所有图片(1)
About This Item
经验公式(希尔记法):
C16H11NO2S
CAS号:
分子量:
281.33
Beilstein:
7930648
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥85% (coupling to amines)
≥98.0% (CHN)
溶解度
ethanol: soluble
螢光
λex 264 nm; λem 313 nm
應用
peptide synthesis
官能基
Fmoc
儲存溫度
−20°C
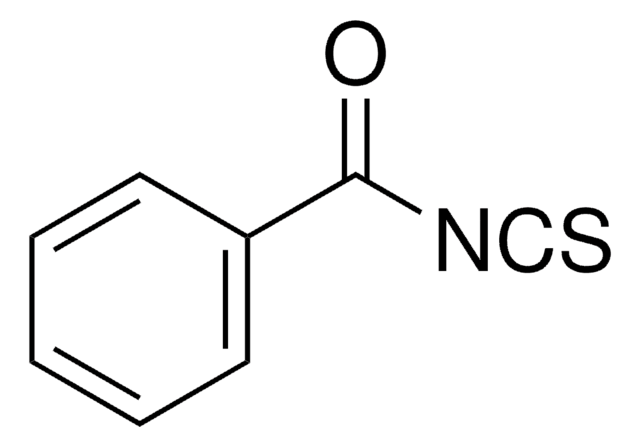
SMILES 字串
O=C(OCC1c2ccccc2-c3ccccc13)N=C=S
InChI
1S/C16H11NO2S/c18-16(17-10-20)19-9-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9H2
InChI 密鑰
DHMYULZVFHHEHE-UHFFFAOYSA-N
應用
Fmoc isothiocyanate can be used:
- As a starting material in the preparation of biologically relevant pharmacophores named N-aryl-N′-carboalkoxy guanidines.
- In one of the intermediate steps for the synthesis of N-aryl-N-thiazolyl derivatives.
- To synthesize cyclic isothiourea derivatives as potent neuropeptide Y (NPY) Y1 receptor antagonists.
- To prepare 2-aminothiazoles, aminobenz-imidazole conjugated thiazoles, and thiazole derived cyclopeptides.
其他說明
由胺类化合物合成取代硫脲类化合物的试剂
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Solid-Phase Synthesis of 2-Aminothiazoles.
Patrick C. Kearney et al.
The Journal of organic chemistry, 63(1), 196-200 (2001-10-25)
Solid-Phase Synthesis of N-Aryl-N′-Carboalkoxy Guanidines by the Mitsunobu Reaction of Fmoc-Guanidines
Robinson DE, et al.
Synthetic Communications, 34(15), 2743-2749 (2004)
Two-step hantzsch based macrocyclization approach for the synthesis of thiazole-containing cyclopeptides
Nefzi A, et al.
The Journal of Organic Chemistry, 75(22), 7939-7941 (2010)
Diversity-oriented synthesis of N-aryl-N-thiazolyl compounds
Nefzi A and Arutyunyan S
Tetrahedron Letters, 51(37), 4797-4800 (2010)
Solid-phase synthesis of 2-aminothiazoles
Kearney PC, et al.
The Journal of Organic Chemistry, 63(1), 196-200 (1998)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门