MSQC4
SILu™Lite SigmaMAb Universal Antibody Standard human
Sinónimos:
IgG1 light, Mass Spectrometry Universal Antibody Standard, SILu™Lite SigmaMAb Universal Antibody Standard human, recombinant IgG1 lambda light antibody, SigmaMAb
About This Item
Productos recomendados
General description
It consists of two identical heavy chains and two identical light chains. The heavy chains and light chains are linked by one disulfide bond. The heavy chains are linked by two disulfide bonds located in a hinge domain. The other 12 cysteine bonds are intramolecularly restricted to six different globular domains. The antibody sequence has been evaluated by intact mass and peptide mapping using four different enzymes: chymotrypsin, Asp-N and Glu-C endoproteinases and trypsin. Sequence coverage of 100% was obtained.
Application
Features and Benefits
Description / Composition / Modification / Average Mass (Da)
Light chain, reduced / C1006H1555N267O333S7 / Pyroglutamic acid (Q) / 22942.2
Heavy chain, reduced / C2181H3393N587O663S16 / (no modification) / 48957.8
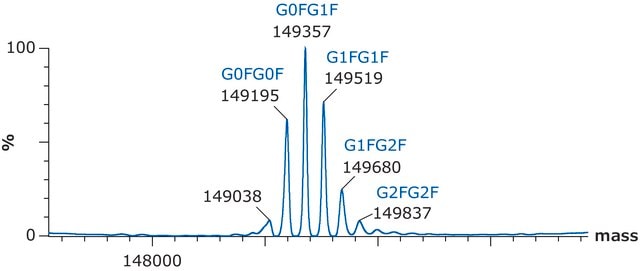
C2237H3485N591O702S16 / G0F / 50403.2
C2243H3495N591O707S16 / G1F / 50565.3
C2249H3505N591O712S16 / G2F / 50727.5
Native intact mass, non-reduced / C6374H9864N1708O1992S46 / 2 X Pyroglutamic acid (Q) / 143767.7
C6486H10048N1716O2070S46 / G0F+G0F / 146658.4
C6492H10058N1716O2075S46 / G0F+G1F / 146820.6
C6498H10068N1716O2080S46 / G1F+G1F / 146982.7
C6504H10078N1716O2085S46 / G1F+G2F / 147144.8
C6510H10088N1716O2090S46 / G2F+G2F / 147307.0
Physical form
Preparation Note
Analysis Note
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKI
GTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRW
APLGAFDIWGQGTMVTVSS|ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
RDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SigmaMab Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIY
DATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGV
VFGGGTKLTVL|GQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTV
AWKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQ
VTHEGSTVEKTVAPTECS
Other Notes
Reconstitute the contents of the vial by adding 500μL of ultrapure water or phosphate buffer, and mixing vigorously. The solubilized product can be further diluted as needed.
Legal Information
Related product
supplement
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Residual presence of host cell proteins (HCPs) in recombinant therapeutic products has considerable clinical safety risks associated with a potential immunological response in patients.
Method development for titer determination of three different monoclonal antibodies - cetuximab, trastuzumab, and universal antibody standard using a Chromolith® WP 300 Protein A column.
Method development for titer determination of three different monoclonal antibodies - cetuximab, trastuzumab, and universal antibody standard using a Chromolith® WP 300 Protein A column.
Method development for titer determination of three different monoclonal antibodies - cetuximab, trastuzumab, and universal antibody standard using a Chromolith® WP 300 Protein A column.
Protocolos
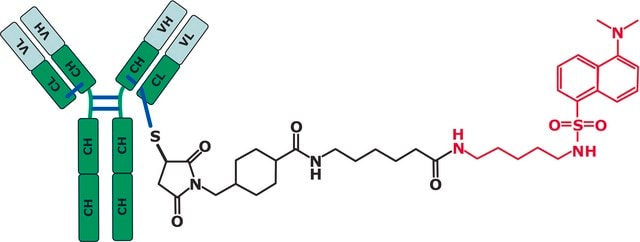
SigmaMab Antibody Drug Conjugate Mimic, is a non-toxic drug mimic utilized as a standard for mass spectrometry and high performance liquid chromatography.
Here we show how LC and MS methods may be optimized using a non-toxic surrogate of the ADC, an “ADC-mimic”, that behaves very similarly to the Cys-linked ADC Adcetris (Seattle Genetics).
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico