All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
bp
142 °C/15 mmHg (lit.)
mp
13 °C (lit.)
density
1.14 g/mL at 25 °C (lit.)
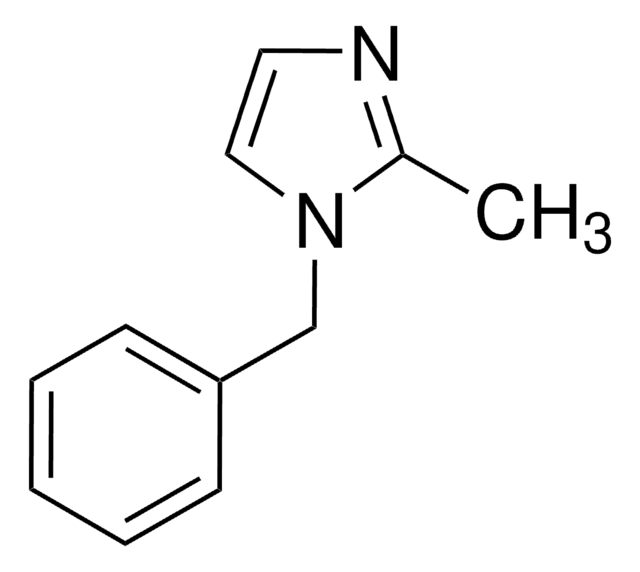
SMILES string
c1ccc(cc1)-n2ccnc2
InChI
1S/C9H8N2/c1-2-4-9(5-3-1)11-7-6-10-8-11/h1-8H
InChI key
SEULWJSKCVACTH-UHFFFAOYSA-N
General description
1-Phenylimidazole is an imidazole derivative. It induces 7-ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout (Oncorhynchus mykiss) hepatocytes. The S(1)→S(0) transition of 1-phenylimidazole has been investigated in a supersonic jet expansion by resonant two-photon ionization. 1-Phenylimidazole is reported to be inhibitor of calmodulin-dependent nitric-oxide synthase from bovine brain and GHs pituitary cells.
Application
1-Phenylimidazole is a suitable reagent used to investigate its effect on the citrulline formation by bovine brain nitric-oxide synthase.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A J Green et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 6(5-6), 523-533 (2001-07-27)
The bioI gene has been sub-cloned and over-expressed in Escherichia coli, and the protein purified to homogeneity. The protein is a cytochrome P450, as indicated by its visible spectrum (low-spin haem iron Soret band at 419 nm) and by the
A Jos et al.
Toxicology in vitro : an international journal published in association with BIBRA, 21(7), 1307-1310 (2007-05-25)
The classical pathway for induction of cytochrome P4501A (CYP1A) by xenobiotics is ligand binding to the aryl hydrocarbon receptor (AhR). However, several studies with mammalian cell systems point out a range of xenobiotics including imidazole derivatives, which are able to
M Murray et al.
Toxicology letters, 25(2), 191-198 (1985-05-01)
Repeated administration of N-phenylimidazole (PI) to rats (3 daily doses of 200 mumol/kg/day) enhanced hepatic microsomal cytochrome P-450 levels (approx. 130%) and aminopyrine N-demethylase (APDM) and aniline p-hydroxylase (APH) activities (approx. 140%); aryl hydrocarbon (benzo[a]pyrene) hydroxylase (AHH) and 7-ethoxycoumarin O-deethylase
M R Anari et al.
Chemical research in toxicology, 9(6), 924-931 (1996-09-01)
Organic hydroperoxides are believed to be primarily detoxified in cells by the GSH peroxidase/GSSG reductase system and activated to cytotoxic radical species by non-heme iron. However, organic hydroperoxides seem to be bioactivated by cytochrome P450 (P450) in isolated hepatocytes as
Filippo Monti et al.
Inorganic chemistry, 54(6), 3031-3042 (2015-03-06)
A series of cationic iridium(III) complexes with two carbene-based cyclometalating ligands and five different N^N bipyridine and 1,10-phenanthroline ancillary ligands is presented. For the first time--in the frame of a rarely studied class of bis(heteroleptic) iridium complexes with two carbene-based
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service











![1-[2-(Trifluoromethyl)phenyl]imidazole](/deepweb/assets/sigmaaldrich/product/structures/150/780/ea7e6b25-7659-422e-868c-8df7fd70d66e/640/ea7e6b25-7659-422e-868c-8df7fd70d66e.png)

