1205003
USP
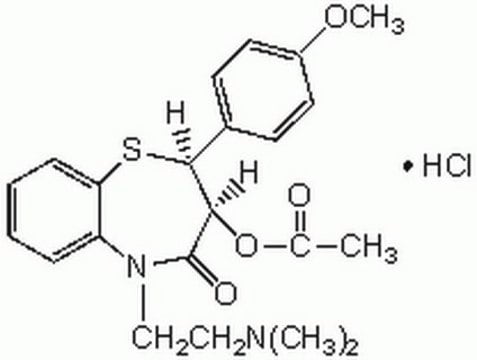
Diltiazem hydrochloride
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
(+)-cis-Diltiazem hydrochloride, (2S,3S)-(+)-cis-3-Acetoxy-5-(2-dimethylaminoethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one hydrochloride, CRD-401
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
diltiazem
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
Chaîne SMILES
Cl.COc1ccc(cc1)[C@@H]2Sc3ccccc3N(CCN(C)C)C(=O)[C@@H]2OC(C)=O
InChI
1S/C22H26N2O4S.ClH/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3;/h5-12,20-21H,13-14H2,1-4H3;1H/t20-,21+;/m1./s1
Clé InChI
HDRXZJPWHTXQRI-BHDTVMLSSA-N
Informations sur le gène
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Exploring the Effectiveness of Carboxymethylated and Crosslinked Albizia procera Gum in Diltiazem Hydrochloride Matrix Tablets: A Comparative Analysis. This study investigates the potential of carboxymethylated and crosslinked Albizia procera gum for use in sustained-release matrix tablets containing Diltiazem hydrochloride, highlighting its viability in pharmaceutical applications (Mukherjee S, Khanam J, 2024).
- Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. While primarily focused on gallic acid, this review mentions Diltiazem hydrochloride in the context of drug interactions and its implications in food safety and health (Xiang Z et al., 2024).
- Dual stimuli-responsive and sustained drug delivery NanoSensoGel formulation for prevention of cisplatin-induced ototoxicity. This research presents a novel NanoSensoGel that could be adapted for Diltiazem hydrochloride, enhancing drug delivery efficiency in clinical settings (Thakur NS et al., 2024).
- A novel potentiometric sensor based on ZnO decorated polyaniline/coal nanocomposite for diltiazem determination. This study develops a new sensor for the precise measurement of Diltiazem levels in pharmaceutical formulations, which is crucial for quality control and regulatory compliance (El Sayed GA et al., 2023).
- The Effect of Topical Nifedipine versus Diltiazem on the Acute Anal Fissure: A Randomized Clinical Trial. This clinical trial evaluates the effectiveness of Diltiazem hydrochloride in treating acute anal fissures, providing evidence of its beneficial applications in proctological disorders (Momayez Sanat Z et al., 2023).
Remarque sur l'analyse
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique