ALD00172
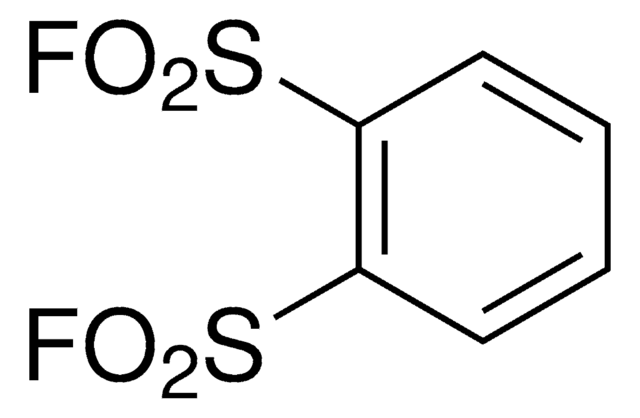
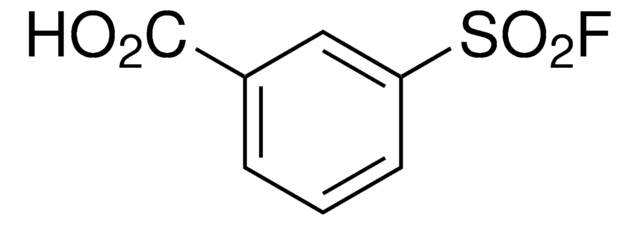
1,3-Benzenedisulfonyl fluoride
95%
About This Item
Produits recommandés
Niveau de qualité
Pureté
95%
Forme
solid
Capacité de réaction
reaction type: click chemistry
Pf
37-42 °C
Chaîne SMILES
FS(C1=CC(S(F)(=O)=O)=CC=C1)(=O)=O
InChI
1S/C6H4F2O4S2/c7-13(9,10)5-2-1-3-6(4-5)14(8,11)12/h1-4H
Clé InChI
VCINFPPPNNZOAD-UHFFFAOYSA-N
Application
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Eye Dam. 1 - Skin Corr. 1B
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
Contenu apparenté
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique