392391
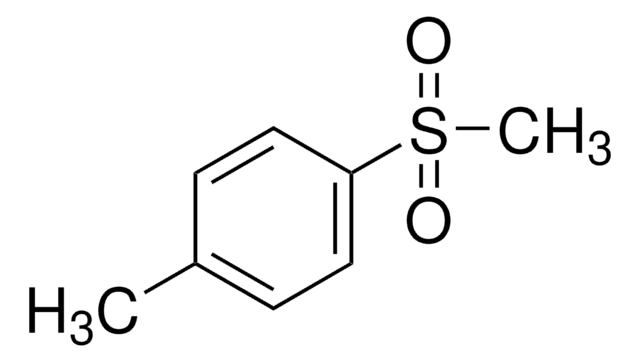
Di-p-tolyl sulfone
99%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
(CH3C6H4)2SO2
Numéro CAS:
Poids moléculaire :
246.32
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
99%
Forme
solid
Pf
158-160 °C (lit.)
Solubilité
chloroform: soluble 25 mg/mL, clear, colorless to yellow
Groupe fonctionnel
sulfone
Chaîne SMILES
Cc1ccc(cc1)S(=O)(=O)c2ccc(C)cc2
InChI
1S/C14H14O2S/c1-11-3-7-13(8-4-11)17(15,16)14-9-5-12(2)6-10-14/h3-10H,1-2H3
Clé InChI
WEAYCYAIVOIUMG-UHFFFAOYSA-N
Description générale
Di-p-tolyl sulfone is a di-p-substituted diaryl sulfone. Its synthesis by the sulfonylation of toluene with p-toluenesulfonic acid (TsOH) in the presence of polystyrene supported aluminium triflate (Ps-Al(OTf)3) catalyst has been reported along with its NMR and IR spectra. The gas-phase heats of formation of di-p-tolyl sulfone has been studied. The kinetics and thermodynamics of sulphuric acid assisted cleavage of di-p-tolyl sulfone has been invesitigated.
Application
Di-p-tolyl sulfone may be used in the synthesis of isomeric p-tolylpyridines (α , β and γ ) by photochemical decomposition.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Studies in the thermochemistry of sulphones. Part 6.-Heats of combustion, fusion, vaporization and atomization of six aromatic and two allylic sulphones.
Mackle H and O'Hare PAG.
Transactions of the Faraday Society, 57, 1521-1526 (1961)
Polystyrene Supported Al (OTf)3: a Stable, Efficient, Selective, and Reusable Catalyst for Sulfonylation of Arenes with Sulfonic Acids.
Boroujeni, KP.
Bull. Korean Chem. Soc., 31(7), 1887-1890 (2010)
Kinetics and thermodynamics of sulfuric acid-mediated cleavage of substituted diaryl sulfones.
Ward RS, et al.
Journal of Surfactants and Detergents, 4(2), 185-190 (2001)
Kazumasa Okamoto et al.
Scientific reports, 10(1), 19823-19823 (2020-11-15)
Dimer radical ions of aromatic molecules in which excess charge is localized in a pair of rings have been extensively investigated. While dimer radical cations of aromatics have been previously produced in the condensed phase, the number of molecules that
Para tolylation of pyridine by photolysis of di-p-tolyl sulfone and related compounds.
Nakabayashi T, et al.
Bulletin of the Chemical Society of Japan, 50(9), 2491-2492 (1977)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique