120812
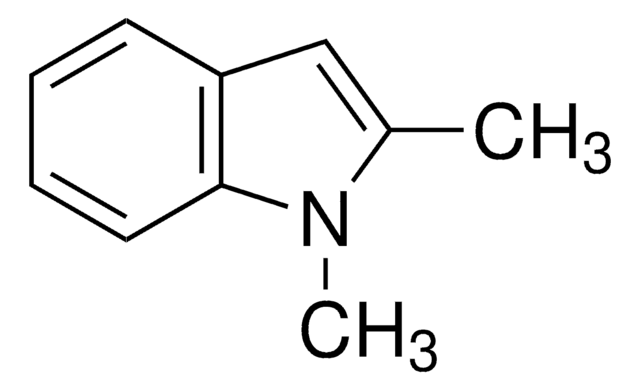
2,3-Dimethylindole
≥97%
Synonyme(s) :
NSC 24936
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C10H11N
Numéro CAS:
Poids moléculaire :
145.20
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Essai
≥97%
Forme
solid
pb
285 °C (lit.)
Pf
105-107 °C (lit.)
Chaîne SMILES
Cc1[nH]c2ccccc2c1C
InChI
1S/C10H11N/c1-7-8(2)11-10-6-4-3-5-9(7)10/h3-6,11H,1-2H3
Clé InChI
PYFVEIDRTLBMHG-UHFFFAOYSA-N
Catégories apparentées
Application
2,3-Dimethylindole has been used to study the mechanism of oxidation of 2,3-dimethylindole by peroxodisulphate and peroxomonosulphate anions to 2-methylindole-2-carbaldehyde. It has been used to study the behaviour of methylindoles in the agilent multimode ion source by atmospheric pressure chemical ionization mass spectrometry.
Reactant for preparation of:
Reactant for:
- Bis(indolyl)methane derivatives
- Potent opioid receptor agonists
- Photorefractive materials
- Prodrugs of the cyclin-dependent kinase (CDK) inhibitor Alsterpaullone
- Dopamine receptors 2/4 (D2/D4) antagonists
- Useful azaspirocyclic building blocks
Reactant for:
- Baylis-Hillman reactions
- Photosensitized Diels-Alder reactions
- Photoinduced electron transfer reactions
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Direct evidence on the mechanism of the oxidation of 2, 3-dimethylindole by inorganic peroxo anions.
Balon M, et al.
The Journal of Organic Chemistry, 58(26), 7469-7473 (1993)
Formation of the Ions of Methylindoles in APCI Mass Spectrometry.
Liu DQ and Sun M.
ISRN Spectroscopy, 2012 (2012)
A I Novaira et al.
Journal of photochemistry and photobiology. B, Biology, 60(1), 25-31 (2001-06-02)
The quenching of anthracene fluorescence by indole, 1,2-dimethylindole (DMI), tryptophan (Trp) and indole 3-acetic acid (IAA) in palmitoyloleoylphosphatidylcholine (POPC) lipid bilayers was investigated. A very efficient quenching of the anthracene fluorescence in the lipid membrane is observed. Stern-Volmer plots are
Resolution of heterogeneous fluorescence from proteins and aromatic amino acids by phase-sensitive detection of fluorescence.
J R Lakowicz et al.
The Journal of biological chemistry, 256(12), 6348-6353 (1981-06-25)
Zhengyin Yan et al.
Analytical chemistry, 80(16), 6410-6422 (2008-07-23)
Constant neutral loss (CNL) and precursor ion (PI) scan have been widely used for the in vitro screening of glutathione conjugates derived from reactive metabolites, but these two methods are only applicable to triple quadrupole or hybrid triple quadrupole mass
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique