PHR1874
Atorvastatin Related Compound I
pharmaceutical secondary standard, certified reference material
Synonyme(s) :
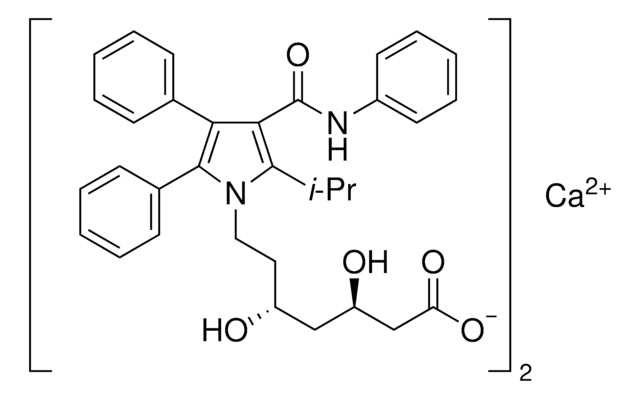
tert-Butyl (4R,6R)-6-[2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1-pyrrolyl]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetate, tert-Butyl 2-((4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxan-4-yl)acetate, (4R,6R)-6-[2-[2-(4-Fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid 1,1-dimethylethyl ester, tert-Butyl 2-[(4R,6R)-6-[2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to USP 1044593
Famille d'API
atorvastatin
CofA (certificat d'analyse)
current certificate can be downloaded
Conditionnement
pkg of 30 mg
Pf
144-148 °C (lit.)
Application(s)
pharmaceutical
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
CC(C)c1c(C(=O)Nc2ccccc2)c(-c3ccccc3)c(-c4ccc(F)cc4)n1CC[C@@H]5C[C@H](CC(=O)OC(C)(C)C)OC(C)(C)O5
InChI
1S/C40H47FN2O5/c1-26(2)36-35(38(45)42-30-16-12-9-13-17-30)34(27-14-10-8-11-15-27)37(28-18-20-29(41)21-19-28)43(36)23-22-31-24-32(47-40(6,7)46-31)25-33(44)48-39(3,4)5/h8-21,26,31-32H,22-25H2,1-7H3,(H,42,45)/t31-,32-/m1/s1
Clé InChI
NPPZOMYSGNZDKY-ROJLCIKYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
Remarque sur l'analyse
Autres remarques
Note de bas de page
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique