PHR1669
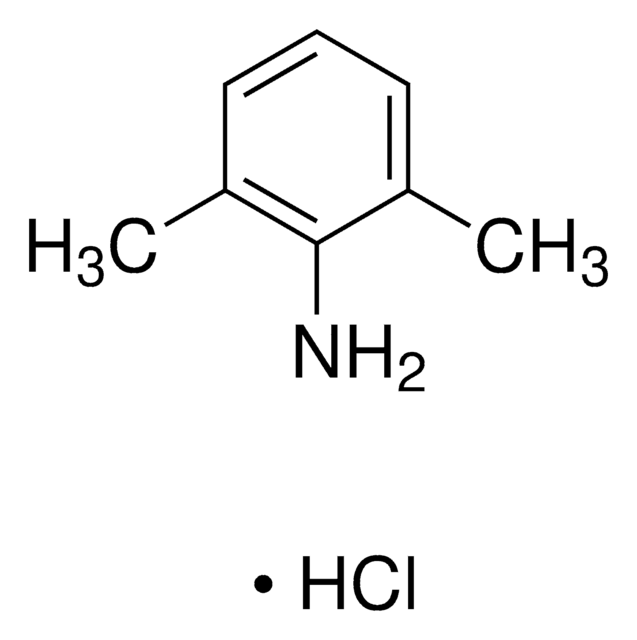
2,6-Dimethylaniline
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyme(s) :
2,6-Dimethylaniline, Lidocaine Impurity A; 2,6 DMA, 2,6-Xylidine, 2-Amino-1,3-dimethylbenzene, 2-Amino-m-xylene
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to Ph. Eur. Y0001575
Pression de vapeur
<0.01 mmHg ( 20 °C)
Famille d'API
lidocaine
CofA (certificat d'analyse)
current certificate can be downloaded
Conditionnement
pkg of 100 mg
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Indice de réfraction
n20/D 1.560 (lit.)
Point d'ébullition
214 °C/739 mmHg (lit.)
Pf
10-12 °C (lit.)
Densité
0.984 g/mL at 25 °C (lit.)
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-30°C
Chaîne SMILES
Cc1cccc(C)c1N
InChI
1S/C8H11N/c1-6-4-3-5-7(2)8(6)9/h3-5H,9H2,1-2H3
Clé InChI
UFFBMTHBGFGIHF-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
The standard is a certified reference material (CRM) qualified with instruments validated according to good manufacturing practices (GMP) using pharmacopeia monograph methods. It is supplied with a comprehensive certificate containing information on traceability assay results, certified purity, homogeneity tests, uncertainty statement, and stability assessment.
Lidocaine Related Compound A is a primary aromatic amine and a major metabolite of the anesthetic lidocaine. It is used as a starting material in the manufacturing of various anesthetics like lidocaine, bupivacaine, mepivacaine, etidocaine, ropivacaine, pyrrocaine, and xylazine.
Application
This pharmaceutical secondary standard can also be used as follows:
- Development of an impurity selective reverse phase-high performance liquid chromatography (RP-HPLC) method to determine dexpanthenol, lidocaine hydrochloride, mepyramine maleate, and their related substances in topical dosage forms
- Testing a selective high-performance liquid chromatography-diode array detection (HPLC-DAD) method, developed for the simultaneous analysis of miconazole nitrate and lidocaine hydrochloride in their combined oral gel dosage form, for its stability-indicating properties
- Evaluation of a high-performance liquid chromatography-diode array detection (HPLC-DAD) procedure― for its stability indicating properties, developed to determine nitrofurazone and lidocaine hydrochloride in their combined dosage form
- Separation of 2,6-Dimethylaniline, its isomeric impurities, and other related impurities by isocratic and reverse-phase ultra-performance liquid chromatographic (UPLC) method
- analyze a binary mixture of lidocaine hydrochloride and cetylpyridinium chloride in presence of lidocaine impurity A by spectrophotometric methods
- determine lidocaine hydrochloride-related substance by analytical methods in pharmaceutical dosage forms
Remarque sur l'analyse
Note de bas de page
Produits recommandés
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
195.8 °F - closed cup
Point d'éclair (°C)
91 °C - closed cup
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique