N9890
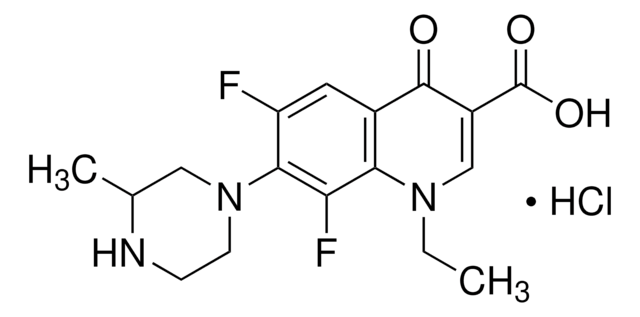
Norfloxacin
analytical standard, ≥98% (TLC)
Synonyme(s) :
Acide 1-éthyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylique
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Agence
EPA 1694
Pureté
≥98% (TLC)
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
clinical testing
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
CCN1C=C(C(O)=O)C(=O)c2cc(F)c(cc12)N3CCNCC3
InChI
1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23)
Clé InChI
OGJPXUAPXNRGGI-UHFFFAOYSA-N
Informations sur le gène
human ... CYP1A2(1544)
rat ... Gabra1(29705)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Mode d′action : inhibe la réplication de l′ADN bactérien
Spectre antimicrobien : bactéries Gram-négatif ; moins efficace contre les bactéries Gram-positif
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique