803340
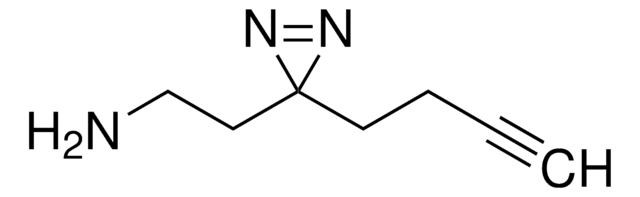
Sulfo-SDA (Sulfo-NHS-Diazirine) (sulfosuccinimidyl 4,4′-azipentanoate)
About This Item
Produits recommandés
Pureté
≥90%
Niveau de qualité
Forme
powder
Poids mol.
327.25
Pertinence de la réaction
reagent type: cross-linking reagent
Conditions de stockage
desiccated
Solubilité
water: soluble
Conditions d'expédition
ambient
Température de stockage
2-8°C
Chaîne SMILES
O=C(C(S(=O)([O-])=O)C1)N(OC(CCC2(N=N2)C)=O)C1=O.[Na+]
InChI
1S/C9H11N3O7S.Na/c1-9(10-11-9)3-2-7(14)19-12-6(13)4-5(8(12)15)20(16,17)18;/h5H,2-4H2,1H3,(H,16,17,18);/q;+1/p-1
Clé InChI
KTYCFZFVXSHAGH-UHFFFAOYSA-M
Catégories apparentées
Description générale
Caractéristiques et avantages
- Water soluble—solubility in aqueous solutions improved by a sulfonate group
- Heterobifunctional—NHS ester group reacts with primary amines at pH 7 to 9 to form covalent amide bonds; diazirine (azipentanoate) group reacts efficiently with any amino acid side chain or peptide backbone upon activation with long-wave UV light (330-370 nm)
- Controllable—two-step chemical crosslinking is activated using common laboratory UV lamps
- Easy to use—these crosslinkers are photo-stable under typical laboratory lighting conditions so there is no need to perform experiments in the dark
- Better than aryl azides—the diazirine photoreactive group has better photostability in normal light than phenyl azide groups of traditional photoreactive crosslinkers, yet the diazirine group is more efficiently activated by long-wave UV light
Attention
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique