291919
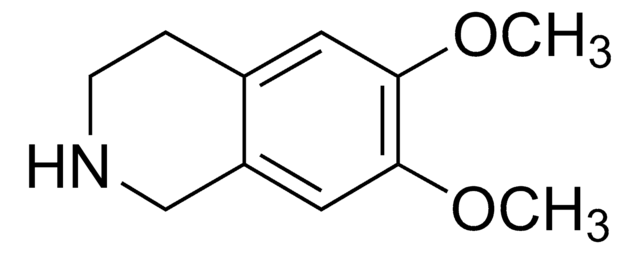
6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H15NO2 · HCl
CAS Number:
Molecular Weight:
229.70
Beilstein:
3634126
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
260-265 °C (lit.)
solubility
soluble 25 mg/mL, clear, colorless (1N NaOH in methanol)
SMILES string
Cl.COc1cc2CCNCc2cc1OC
InChI
1S/C11H15NO2.ClH/c1-13-10-5-8-3-4-12-7-9(8)6-11(10)14-2;/h5-6,12H,3-4,7H2,1-2H3;1H
InChI key
SHOWAGCIRTUYNA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride is also referred as heliamine. It is an alkaloid isolated from mexican cereoid, Backebergia militaris.
Application
6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride has been used as starting material for the synthesis of more complex isoquinolines and quinolizidines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Glusa et al.
Die Pharmazie, 45(6), 408-410 (1990-06-01)
The synthesis of the title compounds starting from 2-Chlormethylbenzdioxan and Tetrahydroisochinolines is presented. Their actions on the platelet aggregation and the inhibition of alpha-adrenoceptors at the isolated rabbit aorta and the vas deferens of the guinea pig were investigated.
R Mata et al.
Journal of pharmaceutical sciences, 69(1), 94-95 (1980-01-01)
Backebergia militaris (Andot) Bravo ex Sánchez Mejorada yielded alkaloid crystals from a fractionated ethanol extract of only 20 g of plant material. The alkaloid was identified as heliamine (6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline) hydrochloride. A second alkaloid, 3,4-dimethoxy-beta-phenethylamine hydrochloride, was crystallized after preparative TLC
Heterocycles, 34, 1857-1857 (1992)
Ken-ichi Umehara et al.
Drug metabolism and disposition: the biological fate of chemicals, 37(7), 1427-1433 (2009-04-11)
(-)-N-{2-[(R)-3-(6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)piperidino]ethyl}-4-fluorobenzamide (YM758), a novel "funny" If current channel (If channel) inhibitor, is developed as a treatment for stable angina and atrial fibrillation. In this study, the pharmacokinetic/pharmacodynamic (PK/PD) relationship after intravenous administration of YM758 to tachycardia-induced dogs was investigated and
The effect of an isoquinoline analogue on voluntary ethanol ingestion in rats.
S Naeger et al.
Proceedings of the Western Pharmacology Society, 34, 35-37 (1991-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service