U4885
UNC0638 hydrate
≥98% (HPLC)
Sinónimos:
2-Cyclohexyl-N-(1-isopropylpiperidin-4-yl)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy) quinazolin-4-amine
Seleccione un Tamaño
142,80 €
Precio de catálogo204,00 €Ahorre 30 %Seleccione un Tamaño
About This Item
142,80 €
Precio de catálogo204,00 €Ahorre 30 %Productos recomendados
Nivel de calidad
Ensayo
≥98% (HPLC)
Formulario
solid
condiciones de almacenamiento
protect from light
color
off-white
solubilidad
DMSO: >10 mg/mL
temp. de almacenamiento
−20°C
cadena SMILES
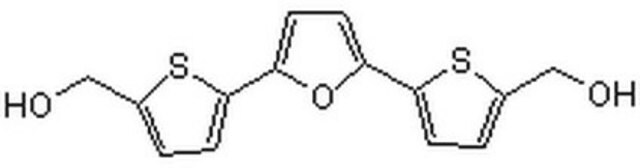
O.COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN4CCCC4)C5CCCCC5
InChI
1S/C30H47N5O2.H2O/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23;/h20-24H,4-19H2,1-3H3,(H,31,32,33);1H2
Clave InChI
LLJGACAJGYXBTL-UHFFFAOYSA-N
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
Características y beneficios
Otras notas
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 2 Oral - Aquatic Chronic 4
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Filtros activos
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico