SAE0090
Beta-galactoside alpha-2,6-sialyltransferase 1
≥300 units/mg protein, ST6GAL1 human recombinant, expressed in HEK 293 cells
Sinónimos:
Alpha 2,6-ST 1, B-cell antigen CD75, CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,6-sialyltransferase 1, ST6Gal I, Sialyltransferase 1
Seleccione un Tamaño
340,00 €
Seleccione un Tamaño
About This Item
340,00 €
Productos recomendados
recombinante
expressed in HEK 293 cells
descripción
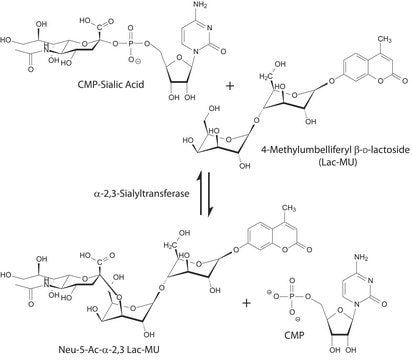
The specific activity of ST6Gal I is measured by its ability to transfer sialic acid from CMP-NANA to asialofetuin.
Ensayo
≥95% (SDS-PAGE)
Formulario
lyophilized powder
actividad específica
≥300 units/mg protein
Condiciones de envío
ambient
temp. de almacenamiento
−20°C
Categorías relacionadas
Descripción general
Acciones bioquímicas o fisiológicas
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity,3 as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.4-5
This recombinant ST6Gal I product can be used to study the mode of action of the enzyme, as well as its potential inhibitors. It can also be used as a glycoengineering tool to modify glycoproteins in vitro.
CMP-N-acetylneuraminate (CMP-sialic acid, CMP-NANA) to the β-D-galactosyl-1,4-N-acetyl-D-glucosaminyl termini on glycoproteins.
Sialic acids are distributed in a variety of glycolipids and glycoproteins. The sialic acid that is added to a galactose (Gal) can be bound either to the hydroxyl attached to carbon-3 of Gal to form an α-2,3 glycosidic linkage, or to the hydroxyl group attached to carbon-6 to form an α-2,6 glycosidic linkage. ST6Gal I generates a α-2,6 linkage of sialic acid on the non-reducing, terminal Galβ1 4GlcNAc residues of oligosaccharides and glycoconjugates.
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity, as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.
Definición de unidad
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Enzymatic glycosyltransferase specificity challenges the one enzyme-one linkage concept.
Filtros activos
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico