L2254
Lipoprotein Lipase from bovine milk
ammonium sulfate suspension, ≥2,000 units/mg protein (BCA)
Sinónimos:
LPL, Phospholipase A1, Diacylglycerol acylhydrolase, Diacylglycerol lipase
About This Item
Productos recomendados
origen biológico
bovine milk
Nivel de calidad
formulario
ammonium sulfate suspension
actividad específica
≥2,000 units/mg protein (BCA)
temp. de almacenamiento
2-8°C
Descripción general
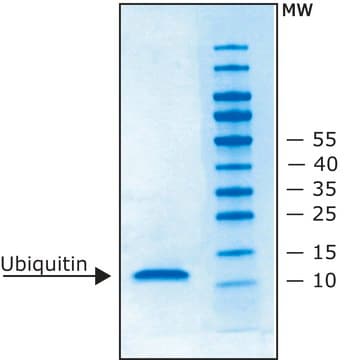
Lipoprotein Lipase (LPL) from bovine milk is a glycoprotein. It exists as a homodimer and comprises two N-linked oligosaccharides. It is heat-labile.Lipoprotein lipase is an enzyme found on the surface of vascular endothelial cells, where it is anchored to capillary walls. It is mainly present in adipose tissue, heart, and muscle tissue. It is synthesized by extrahepatic tissues, particularly adipocytes, and the gene encoding the protein is situated on chromosome 8p22.
Aplicación
- as a supplement to test its effect on DiI (1,1′-dioctadecyl-3,3,3′-tetramethyl-indocarbocyanine perchlorate)- very-low-density lipoprotein (VLDL) uptake in breast cancer MDA-MB-231 cells.
- to treat human brain microvascular endothelial cells (HBMECs) for the lipolysis of triglyceride-rich lipoproteins (TGRL).
- to test its effect on gene expression in normal human astrocytes.
- in primary hepatocyte isolation and lipoprotein binding to identify Sulf2 inhibition in T2DM mice for improving diabetic dyslipidemia.
- in transforming growth factor-beta (TGF-β1) immunoassay to test if the TGF-β signaling system regulates the up-regulation and activation of activating transcription factor 3 (ATF3) in human aortic endothelial cells (HAEC) induced by lipolysis products.
- in human TGRL isolation.
- in in vitro lipolysis assay with HSPG-bound LPL, to investigate the effect of human apoE2 (Lys146→Gln) on lipoprotein metabolism.
- in hydrolysis of triglycerides.
- in developing in vitro model of gastrointestinal digestion to investigate the effects of chlorophyll on lipid digestion.
Acciones bioquímicas o fisiológicas
Definición de unidad
Forma física
Nota de preparación
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Lipid Induced Insulin Resistance
Instructions for working with enzymes supplied as ammonium sulfate suspensions
Protocolos
Lipoprotein lipase (LPL) hydrolyzes triglycerides associated with VLDL.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico