03449
N-(3-dimetilaminopropilo)-N′-etilcarbodiimida hydrochloride
≥99.0% (AT)
Sinónimos:
N-etil-N′-(3-dimetilaminopropil)carbodiimida hydrochloride, EDAC, EDC, EDC hydrochloride, WSC hydrochloride
Seleccione un Tamaño
87,90 €
Seleccione un Tamaño
About This Item
87,90 €
Productos recomendados
Nivel de calidad
Ensayo
≥99.0% (AT)
Formulario
powder
técnicas
bioconjugation: suitable
mp
110-115 °C (lit.)
112-116 °C
solubilidad
H2O: soluble 0.2 g/L
temp. de almacenamiento
−20°C
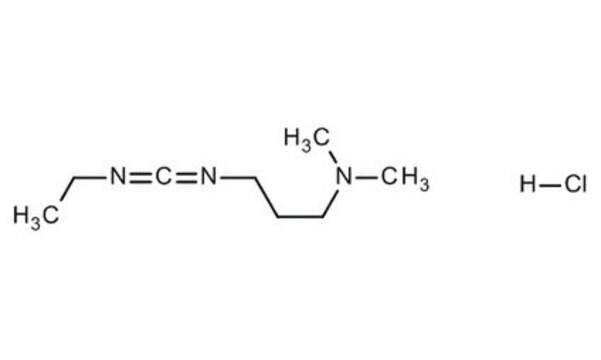
cadena SMILES
Cl.CCN=C=NCCCN(C)C
Cl.CCN=C=NCCCN(C)C
InChI
1S/C8H17N3.ClH/c1-4-9-8-10-6-5-7-11(2)3;/h4-7H2,1-3H3;1H
Clave InChI
FPQQSJJWHUJYPU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Furthermore, EDAC HCl serves as a biomolecule bridge, acting as a crosslinker that connects amine-reactive NHS-esters of biomolecules to carboxyl groups. This technique proves invaluable in protein conjugation, enabling the creation of hybrid molecules with novel properties and functions. The underlying mechanism involves EDAC HCl′s reaction with a carboxyl group, forming an unstable intermediate that actively seeks an amine partner. The delicate balance of this reaction underscores the importance of optimizing conditions for efficient conjugation. The assistance of N-hydroxysuccinimide (NHS) further enhances EDAC HCl′s capabilities by stabilizing the intermediate and enabling two-step conjugation procedures. This additional feature provides greater flexibility and control, particularly when dealing with complex biomolecules.
Aplicación
Acciones bioquímicas o fisiológicas
Características y beneficios
Otras notas
producto comparable
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Órganos de actuación
Stomach,large intestine,lymph node
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Professor Aran discusses engineering graphene-based materials through careful functionalization, enabling diverse applications.
Filtros activos
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico








![1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide](/deepweb/assets/sigmaaldrich/product/structures/414/134/4eb9c126-d7f9-4e12-9e3a-95cb077824fd/640/4eb9c126-d7f9-4e12-9e3a-95cb077824fd.png)



