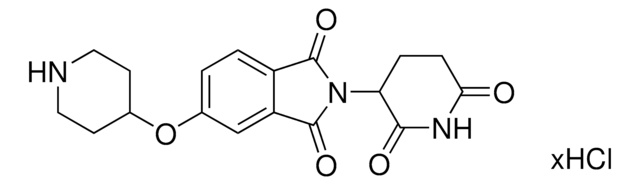
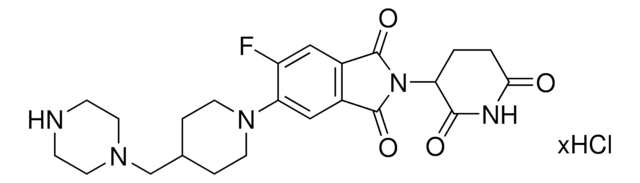
923907
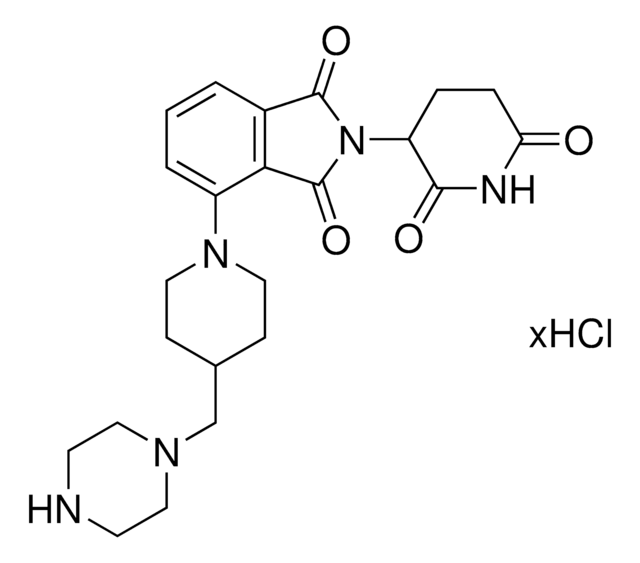
6F,C5-Pomalidomide-piperazine-piperidine-4-carbothioamide hydrochloride
Sinónimos:
2-(2,6-Dioxopiperidin-3-yl)-5-fluoro-6-(4-(piperidine-4-carbonothioyl)piperazin-1-yl)isoindoline-1,3-dione hydrochloride, Crosslinker–E3 ligase ligand conjugate, Protein degrader building block
About This Item
Productos recomendados
ligand
6F,C5-Pomalidomide
Nivel de calidad
idoneidad de la reacción
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
grupo funcional
amine
temp. de almacenamiento
2-8°C
cadena SMILES
O=C1C(N2C(C(C=C(F)C(N3CCN(C(C4CCNCC4)=S)CC3)=C5)=C5C2=O)=O)CCC(N1)=O.Cl
Aplicación
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Otras notas
Información legal
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Repr. 1B
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico