919888
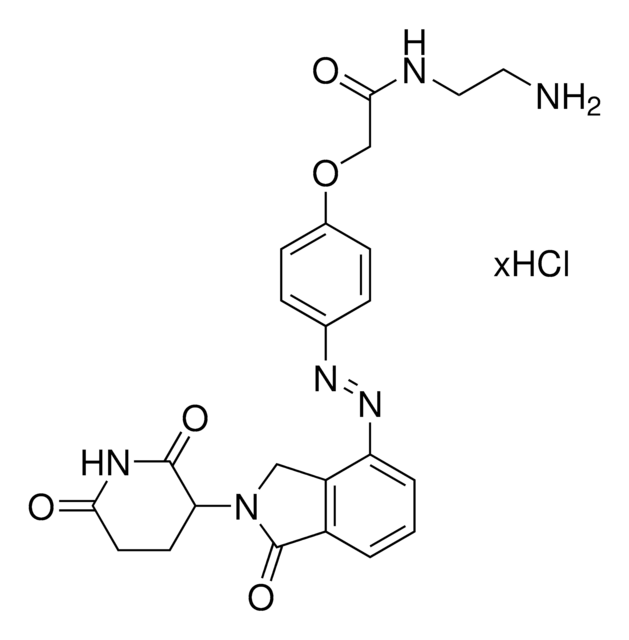
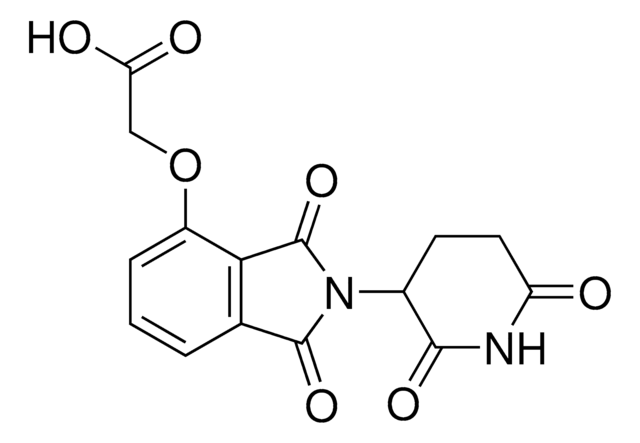
Thalidomide-Photoswitch3-NH2 hydrochloride
≥95%
Sinónimos:
(E)-N-(4-((4-Aminophenyl)diazenyl)phenyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamide hydrochloride, Photoswitchable protein degrader building block for PROTAC®
About This Item
Productos recomendados
ligand
thalidomide
Nivel de calidad
Análisis
≥95%
formulario
solid
idoneidad de la reacción
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
grupo funcional
amine
temp. de almacenamiento
2-8°C
cadena SMILES
O=C1N(C2C(NC(CC2)=O)=O)C(C3=C1C=CC=C3OCC(NC4=CC=C(/N=N/C5=CC=C(N)C=C5)C=C4)=O)=O.Cl
Categorías relacionadas
Aplicación
Suggested wavelengths for photoswitching:
- Switch to cis isomer: 390 nm (380-400 nm)
- Switch to trans isomer (thermally more stable isomer): >450 nm
Browse our full offering of degrader building blocks that streamlines the synthesis of degrader libraries.
Learn more:
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Portal: Building PROTAC Degraders for Targeted Protein Degradation
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Otras notas
Información legal
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico