726583
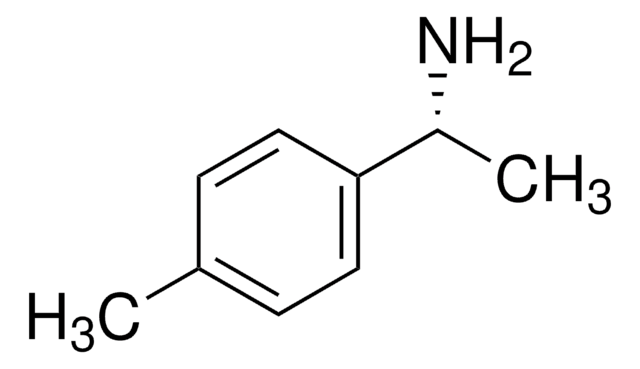
(S)-(−)-α-Methylbenzylamine
ChiPros®, produced by BASF, ≥99.0%
Sinónimos:
(S)-(-)-alpha-Methylbenzylamine, (S)-(−)-1-Phenylethylamine
About This Item
Productos recomendados
grado
produced by BASF
presión de vapor
0.5 mmHg ( 20 °C)
Ensayo
≥99.0% (GC)
≥99.0%
Formulario
liquid
pureza óptica
enantiomeric excess: ≥99.0%
índice de refracción
n20/D 1.526 (lit.)
bp
187 °C (lit.)
densidad
0.94 g/mL at 25 °C (lit.)
grupo funcional
amine
phenyl
cadena SMILES
C[C@H](N)c1ccccc1
InChI
1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3/t7-/m0/s1
Clave InChI
RQEUFEKYXDPUSK-ZETCQYMHSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- The first example of a diastereoselective thio-Ugi reaction: a new synthetic approach to chiral imidazole derivatives.: This research presents a novel diastereoselective thio-Ugi reaction, enabling the synthesis of chiral imidazole derivatives, which are essential in asymmetric synthesis and pharmaceutical intermediates (Gulevich et al., 2007).
- Synthesis and serotonin receptor affinities of a series of enantiomers of alpha-methyltryptamines: evidence for the binding conformation of tryptamines at serotonin 5-HT1B receptors.: This study investigates the synthesis and serotonin receptor affinities of alpha-methyltryptamine enantiomers, contributing to the understanding of chiral compounds in medicinal chemistry (Nichols et al., 1988).
Información legal
Palabra de señalización
Danger
Frases de peligro
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Skin Corr. 1B
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
158.0 °F - closed cup
Punto de inflamabilidad (°C)
70 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
Filtros activos
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico