240761
1-Hexene
≥99%
Sinónimos:
1-n-Hexene
About This Item
Productos recomendados
densidad de vapor
3 (vs air)
presión de vapor
155 mmHg ( 21.1 °C)
Análisis
≥99%
formulario
liquid
temp. de autoignición
487 °F
índice de refracción
n20/D 1.388 (lit.)
bp
60-66 °C (lit.)
densidad
0.678 g/mL at 25 °C (lit.)
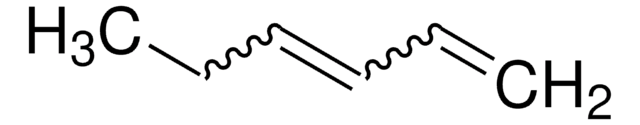
cadena SMILES
CCCCC=C
InChI
1S/C6H12/c1-3-5-6-4-2/h3H,1,4-6H2,2H3
Clave InChI
LIKMAJRDDDTEIG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
- 1-Hexene in High Molecular Weight Polymer Synthesis: The synthesis of high molecular weight copolymers from 1-hexene and methyl acrylate using Lewis acid catalysts showcased advanced applications in materials science, specifically in developing durable and versatile polymer materials (Wan et al., 2024).
- 1-Hexene′s Role in Proton-Exchange Membrane Enhancement: Utilizing 1-hexene in the structural characterization and enhancement of physicochemical properties of functionally porous proton-exchange membranes highlights its critical role in improving energy efficiency and performance in fuel cell technologies (Ponomar et al., 2024).
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Asp. Tox. 1 - Flam. Liq. 2
Riesgos supl.
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
-13.0 °F - closed cup
Punto de inflamabilidad (°C)
-25.0 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico