W398403
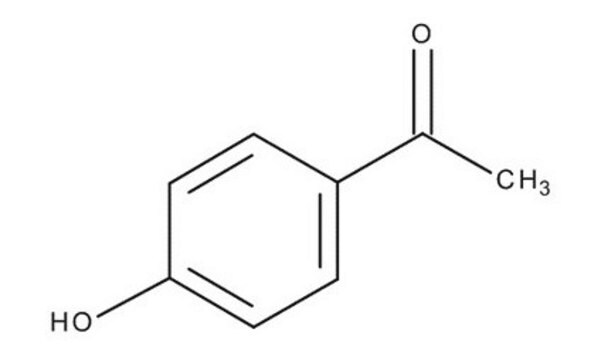
4-Hydroxybenzaldehyde
≥97%, FG
Sinónimos:
p-Hydroxybenzaldehyde
About This Item
Productos recomendados
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 110
assay
≥97%
mp
112-116 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
organoleptic
almond; balsamic; nutty; sweet
SMILES string
[H]C(=O)c1ccc(O)cc1
InChI
1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H
InChI key
RGHHSNMVTDWUBI-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Dual action of benzaldehydes: Inhibiting quorum sensing and enhancing antibiotic efficacy for controlling Pseudomonas aeruginosa biofilms.: This paper highlights the dual functionality of benzaldehyde derivatives in inhibiting bacterial communication and boosting antibiotic efficiency, offering promising strategies against biofilm-associated infections (Borges A et al., 2024).
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
345.2 °F
flash_point_c
174 °C
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico