89231
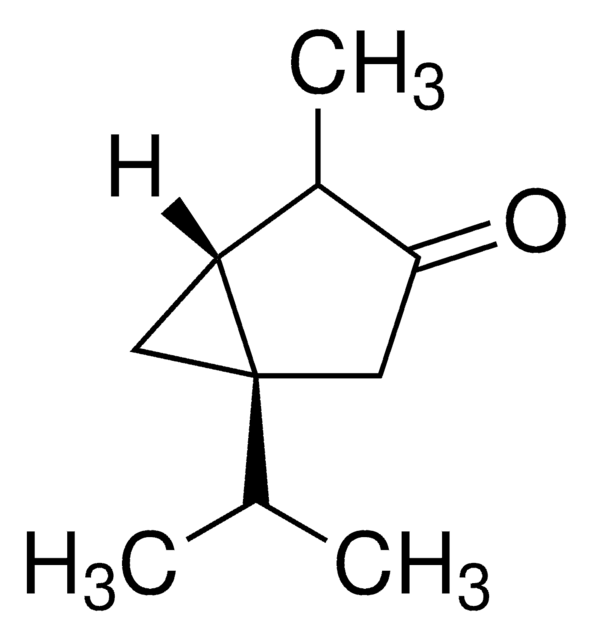
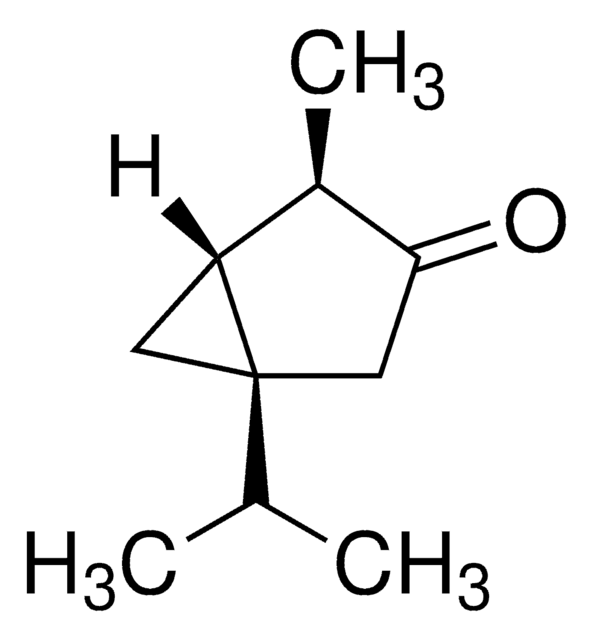
(−)-α-Thujone
≥96.0% (GC)
Sinónimos:
(-)-alpha-Thujone, (1S,4R)-1-Isopropyl-4-methylbicyclo[3.1.0]hexan-3-one
About This Item
Productos recomendados
assay
≥96.0% (GC)
form
liquid
optical activity
[α]20/D −19.0±2.0°, neat
refractive index
n20/D 1.450
density
0.914 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2
InChI
1S/C10H16O/c1-6(2)10-4-8(10)7(3)9(11)5-10/h6-8H,4-5H2,1-3H3/t7-,8-,10+/m1/s1
InChI key
USMNOWBWPHYOEA-MRTMQBJTSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- A six-step total synthesis of α-thujone and d6-α-thujone, enabling facile access to isotopically labelled metabolites: This study presents a concise method for synthesizing α-thujone, useful for creating isotopically labeled derivatives for research purposes (Thamm et al., 2016).
- Enhancement of CD3AK cell proliferation and killing ability by α-thujone: Investigates α-thujone′s ability to enhance the proliferation and cytotoxic activity of CD3AK cells, suggesting potential immunological applications (Zhou et al., 2016).
- α-Thujone exhibits an antifungal activity against F. graminearum by inducing oxidative stress, apoptosis, epigenetics alterations and reduced toxin synthesis: Demonstrates the antifungal effects of α-thujone against Fusarium graminearum, which could make it a valuable alternative to traditional fungicides (Teker et al., 2021).
Biochem/physiol Actions
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
147.2 °F - closed cup
flash_point_c
64 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico