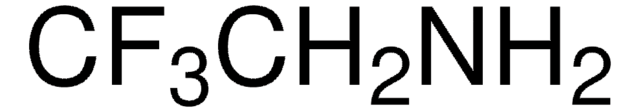549797
Chlorure d'acryloyle
97.0%, contains <210 ppm MEHQ as stabilizer
Synonyme(s) :
Chlorure de 2-propénoyle
About This Item
Produits recommandés
Densité de vapeur
>1 (vs air)
Pression de vapeur
1.93 psi ( 20 °C)
Pureté
97.0%
Contient
<210 ppm MEHQ as stabilizer
Indice de réfraction
n20/D 1.435 (lit.)
Point d'ébullition
72-76 °C (lit.)
Densité
1.114 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
ClC(=O)C=C
InChI
1S/C3H3ClO/c1-2-3(4)5/h2H,1H2
Clé InChI
HFBMWMNUJJDEQZ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Acrylic polymers via radical polymerization or copolymerization. These acrylic polymers can be tailored to possess the desired properties for biomedical coatings, including biocompatibility, adhesion to the device surface, and durability.
- Poly(styrene-co-acryloyl chloride) copolymer by crosslinked networks with styrene. The resulting crosslinked polymer can then be functionalized or modified by various chemical reactions to introduce specific properties or functionalities desired for the application as a polymer support or an electrophilic scavenger resin.
- Acrylamide-modified chitosan.
- Ulvan acrylate macromer via esterification of hydroxyl groups of polysaccharides. This macromer can be used to prepare ulvan-based thermosensitive hydrogels.
- Degradable peptide cross-linker by the acrylation of the amine groups of lysine residues and glutamine within peptide sequences.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 1 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Met. Corr. 1 - Skin Corr. 1A
Risques supp
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
30.2 °F
Point d'éclair (°C)
-1 °C
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique