244759
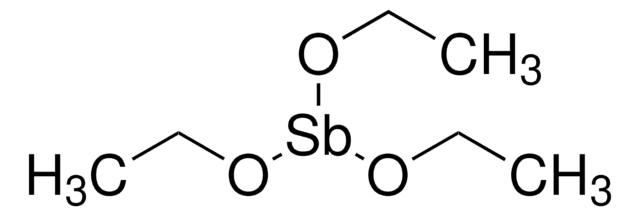
Titanium(IV) ethoxide
technical grade
Synonyme(s) :
Tetraethyl orthotitanate, Tetraethyl titanate
About This Item
Produits recommandés
Qualité
technical grade
Niveau de qualité
Forme
liquid
Composition
Ti, 19.0-21.0% gravimetric
Pertinence de la réaction
core: titanium
reagent type: catalyst
Impuretés
~20% Tetraisopropyl orthotitanate
Indice de réfraction
n20/D 1.505 (lit.)
Point d'ébullition
150-152 °C/10 mmHg (lit.)
Densité
1.088 g/mL at 25 °C (lit.)
Chaîne SMILES
CCO[Ti](OCC)(OCC)OCC
InChI
1S/4C2H5O.Ti/c4*1-2-3;/h4*2H2,1H3;/q4*-1;+4
Clé InChI
JMXKSZRRTHPKDL-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- in the sol-gel synthesis of free-standing ferroelectric lead zirconate titanate nanoparticles
- spherical titania cores, produced either by hydrolysis of Ti(IV) ethoxide droplets in contact with water vapor or
- by preadsorption of the titanium alkoxides into/onto the clay mineral platelets and their subsequent hydrolysis and calcination.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Flam. Liq. 3
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
107.6 °F - closed cup
Point d'éclair (°C)
42 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique