Wichtige Dokumente
860345P
Avanti
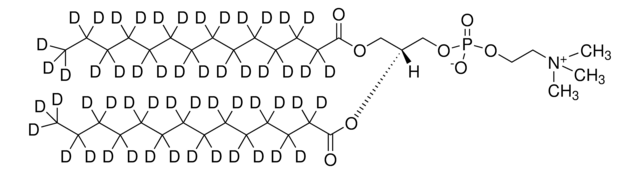
14:0 PC-d54
1,2-dimyristoyl-d54-sn-glycero-3-phosphocholine, powder
Synonym(e):
DMPC-D54
Größe auswählen
Größe auswählen
About This Item
Empfohlene Produkte
Assay
>99% (TLC)
Form
powder
Verpackung
pkg of 1 × 10 mg (860345P-10mg)
pkg of 1 × 100 mg (860345P-100mg)
pkg of 1 × 200 mg (860345P-200mg)
pkg of 1 × 25 mg (860345P-25mg)
Hersteller/Markenname
Avanti Research™ - A Croda Brand 860345P
Versandbedingung
dry ice
Lagertemp.
−20°C
SMILES String
[O-]P(OCC[N+](C)(C)C)(OC[C@]([H])(OC(C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])[2H])=O)COC(C([2H])([2H])C([2H])([2H])C([2H])([2H
InChI
1S/C36H72NO8P/c1-6-8-10-12-14-16-18-20-22-24-26-28-35(38)42-32-34(33-44-46(40,41)43-31-30-37(3,4)5)45-36(39)29-27-25-23-21-19-17-15-13-11-9-7-2/h34H,6-33H2,1-5H3/t34-/m1/s1/i1D3,2D3,6D2,7D2,8D2,9D2,10D2,11D2,12D2,13D2,14D2,15D2,16D2,17D2,18D2,19D2,20D2,21D2,22D2,23D2,24D2,25D2,26D2,27D2,28D2,29D2
InChIKey
CITHEXJVPOWHKC-RPLUSTTMSA-N
Allgemeine Beschreibung
Anwendung
Verpackung
Rechtliche Hinweise
auch häufig zusammen mit diesem Produkt gekauft
Lagerklassenschlüssel
11 - Combustible Solids
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
The critical micelle concentration (CMC) can be approximately defined as the lipid monomer concentration at which appreciable amounts (>5% of total) of micellar aggregates first begin to appear in the equilibrium: nM1<=>Mn
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.










![14:0 Cardiolipin (sodium salt) 1′,3′-bis[1,2-dimyristoyl-sn-glycero-3-phospho]-glycerol (sodium salt), powder](/deepweb/assets/sigmaaldrich/product/images/390/523/992bed4e-3608-4209-aa6e-fa9206a4a31b/640/992bed4e-3608-4209-aa6e-fa9206a4a31b.jpg)


