Wichtige Dokumente
A75900
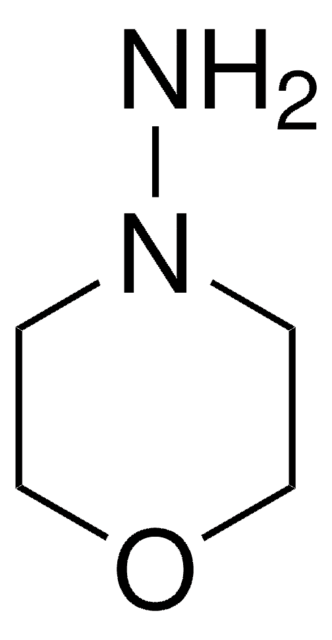
1-Aminopiperidin
97%
Synonym(e):
Pentamethylenehydrazine, Piperidin-1-ylamine
About This Item
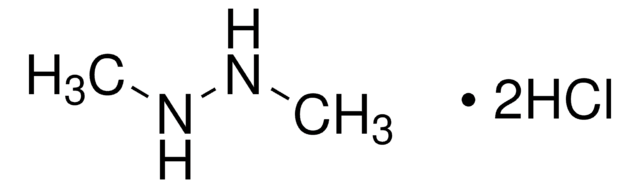
Empfohlene Produkte
Assay
97%
Form
liquid
Brechungsindex
n20/D 1.475 (lit.)
bp
146 °C/730 mmHg (lit.)
Dichte
0.928 g/mL at 25 °C (lit.)
SMILES String
NN1CCCCC1
InChI
1S/C5H12N2/c6-7-4-2-1-3-5-7/h1-6H2
InChIKey
LWMPFIOTEAXAGV-UHFFFAOYSA-N
Anwendung
- CB1 cannabinoid receptor ligands†
- Hydrazones†
- Tetrahydronaphthalene derivatives affecting proliferation and nitric oxide production in LPS activated RAW 264.7 macrophages
- Phosphorus(V) hydrazines
Sonstige Hinweise
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 3
Flammpunkt (°F)
96.8 °F - closed cup
Flammpunkt (°C)
36 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Aktive Filter
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.