08012
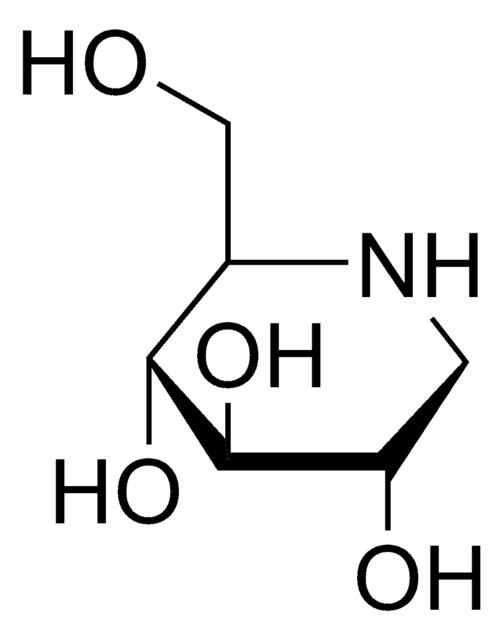
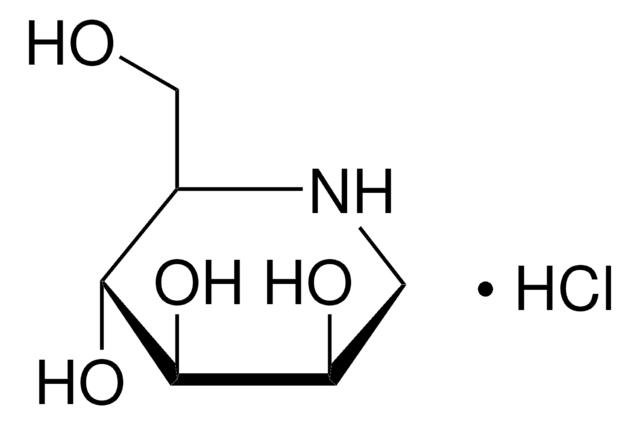
1-Deoxynojirimycin
analytical standard
Synonym(s):
1,5-Dideoxy-1,5-imino-D-sorbitol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H13NO4
CAS Number:
Molecular Weight:
163.17
Beilstein:
3588039
MDL number:
UNSPSC Code:
85151701
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥95.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
format
neat
SMILES string
OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1
InChI key
LXBIFEVIBLOUGU-JGWLITMVSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Deoxynojirimycin is a polyhydroxypiperidine alkaloid and glucose analog, that has an -NH group substituting the O atom in the pyranose ring. It shows a significant α-glucosidase inhibitory effect in addition to antioxidant, antimicrobial, antidiabetic, antitumor, and anti-inflammatory behavior. It is commonly found in the roots of mulberry trees.
Application
DNJ may be used as a reference standard for the determination of DNJ in:
- Mulberry leaves by high-performance liquid chromatography (HPLC) equipped with evaporative light scattering detector (ELSD) as well as direct analysis in real-time (DART) ionization source coupled with triple quadrupole tandem mass spectrometry (MS/MS).
- Silkworms by hydrophilic interaction chromatography (HILIC) with MS/MS operating on multiple reaction monitoring (MRM) mode.
- Mulberry leaves of 132 varieties belonging to nine Morus species following its derivatization with fluorenylmethyl chloroformate (FMOC-Cl) by high-performance liquid chromatography (HPLC) combined with photodiode array (PDA) detector
- Development of an analytical method based on hydrophilic interaction chromatography (HILIC) coupled with tandem mass spectrometry (MS/MS) in positive ion electrospray ionization mode in the leaves of 32 cultivars of different Morus species
- HPLC method-based separation and quantification in the ethanol extracts of the leaves of Morus alba L. and Morus nigra with fluorimetric detection following pre-column derivatization with 9-fluorenylmethyl chloroformate
- 146 varieties of mulberry fruits from nine genera by HPLC in combination with UV-Vis dual-wavelength detection
- Human plasma by hybrid quadrupole/linear ion trap tandem mass spectrometry (QTRAP MS/MS).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Katsumi Higaki et al.
Future medicinal chemistry, 5(13), 1551-1558 (2013-09-13)
A growing body of evidence suggests that misfolding of a mutant protein followed by its aggregation or premature degradation in the endoplasmic reticulum is one of the main mechanisms that underlie inherited neurodegenerative diseases, including lysosomal storage diseases. Chemical or
Bi-Qing Li et al.
PloS one, 8(6), e65207-e65207 (2013-06-14)
Acquired immune deficiency syndrome (AIDS) is a severe infectious disease that causes a large number of deaths every year. Traditional anti-AIDS drugs directly targeting the HIV-1 encoded enzymes including reverse transcriptase (RT), protease (PR) and integrase (IN) usually suffer from
Daisuke Kitano et al.
Cardiovascular diabetology, 12, 92-92 (2013-06-20)
Hyperglycemia, a risk factor for development of cardiovascular disease, causes endothelial dysfunction. Alpha-glucosidase inhibitors (α-GIs) improve postprandial hyperglycemia (PPHG) and may have favorable effects on associated cardiovascular disease. Effects of α-GIs in patients with acute coronary syndrome (ACS) and PPHG
Danielle te Vruchte et al.
The Journal of clinical investigation, 124(3), 1320-1328 (2014-02-04)
Lysosomal storage disorders (LSDs) occur at a frequency of 1 in every 5,000 live births and are a common cause of pediatric neurodegenerative disease. The relatively small number of patients with LSDs and lack of validated biomarkers are substantial challenges
Quantitative determination of 1-deoxynojirimycin in mulberry leaves using liquid chromatography-tandem mass spectrometry
Nuengchamnong N, et al.
Journal of Pharmaceutical and Biomedical Analysis, 44(4), 853-858 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service