1138008
USP
Clindamycin phosphate
United States Pharmacopeia (USP) Reference Standard
Synonyma:
Clindamycin 2-phosphate, Clindamycin 2-dihydrogen phosphate
About This Item
Doporučené produkty
grade
pharmaceutical primary standard
API family
clindamycin
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
−20°C
SMILES string
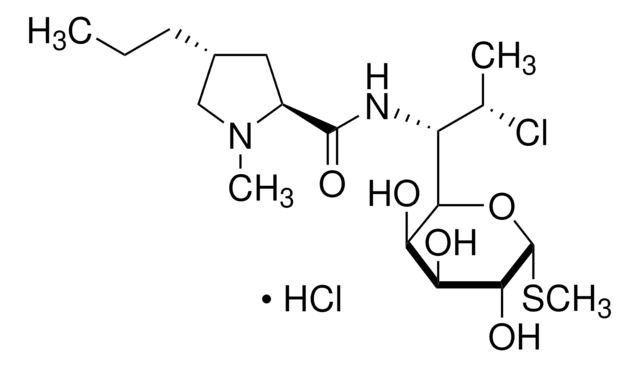
CCC[C@@H]1C[C@H](N(C)C1)C(=O)NC([C@H](C)Cl)C2O[C@H](SC)[C@H](OP(O)(O)=O)[C@@H](O)[C@H]2O
InChI
1S/C18H34ClN2O8PS/c1-5-6-10-7-11(21(3)8-10)17(24)20-12(9(2)19)15-13(22)14(23)16(18(28-15)31-4)29-30(25,26)27/h9-16,18,22-23H,5-8H2,1-4H3,(H,20,24)(H2,25,26,27)/t9-,10+,11-,12?,13+,14-,15?,16+,18+/m0/s1
InChI key
UFUVLHLTWXBHGZ-MWBQRTRKSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
- Clindamycin Phosphate Gel
- Clindamycin Phosphate Vaginal Cream
- Clindamycin Phosphate Topical Suspension
- Clindamycin Phosphate Vaginal Inserts
- Clindamycin Phosphate Topical Solution
- Clindamycin Injection
Biochem/physiol Actions
Mode of Action: Inhibits protein synthesis in bacterial by binding the 50s ribosomal subunit.
Other Notes
Analysis Note
related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Lact. - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.