SMB01003
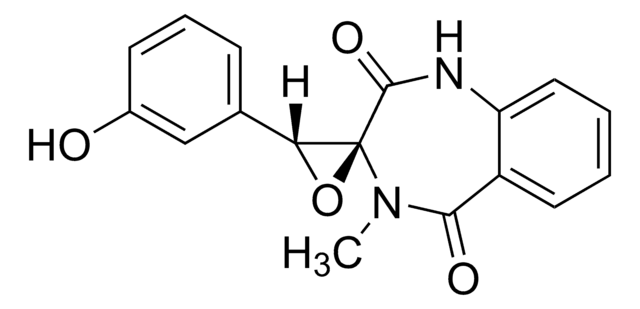
Cyclopenin
≥90% (LC/MS-ELSD)
Synonyma:
(-)-Cyclopenin, (-)-Cyclopenine, 4-methyl-3′-phenylspiro[1H-1,4-benzodiazepine-3,2′-oxirane]-2,5-dione
About This Item
Doporučené produkty
biological source
plant
assay
≥90% (LC/MS-ELSD)
form
solid
mol wt
294.3
solubility
water: slightly soluble
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
−20°C
InChI
1S/C17H14N2O3/c1-19-15(20)12-9-5-6-10-13(12)18-16(21)17(19)14(22-17)11-7-3-2-4-8-11/h2-10,14H,1H3,(H,18,21)
InChI key
APLKWZASYUZSBL-UHFFFAOYSA-N
General description
Application
Biochem/physiol Actions
Features and Benefits
- High quality compound suitable for multiple research applications
- Compatible with HPLC and mass spectrometry techniques
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.