S3381
Sodium stearate
≥99%
Synonyma:
Octadecanoic acid sodium salt, Stearic acid sodium salt
About This Item
Doporučené produkty
biological source
plant (vegetable)
Quality Level
assay
≥99%
form
powder
functional group
ester
lipid type
saturated FAs
shipped in
ambient
storage temp.
2-8°C
SMILES string
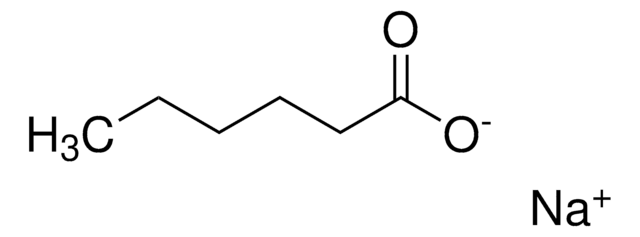
[Na+].CCCCCCCCCCCCCCCCCC([O-])=O
InChI
1S/C18H36O2.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20;/h2-17H2,1H3,(H,19,20);/q;+1/p-1
InChI key
RYYKJJJTJZKILX-UHFFFAOYSA-M
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
Application
- Stability test of novel combined formulated dry powder inhalation system containing antibiotic: physical characterization and in vitro-in silico lung deposition results.: This study investigates a novel dry powder inhalation system formulated with an antibiotic and sodium stearate. It explores the physical characterization and lung deposition efficiency, demonstrating the potential for enhanced drug delivery to the lungs (Benke et al., 2019).
- Protective effect of sodium stearate on the moisture-induced deterioration of hygroscopic spray-dried powders.: This research highlights the role of sodium stearate in protecting hygroscopic spray-dried powders from moisture-induced degradation, which is crucial for maintaining the stability and efficacy of pharmaceutical products (Yu et al., 2018).
- Dissolution of a poorly water-soluble drug dry coated with magnesium and sodium stearate.: This study examines the use of sodium stearate in enhancing the dissolution rates of poorly water-soluble drugs, which is critical for improving drug bioavailability (Tay et al., 2012).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.