P7605
Anti-PARP antibody produced in rabbit
affinity isolated antibody, buffered aqueous solution
Synonyma:
Anti-Poly[ADP-ribose] Polymerase
About This Item
Doporučené produkty
biological source
rabbit
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
mol wt
antigen 116 kDa
species reactivity
human
technique(s)
indirect immunofluorescence: 1:100 using cultured MCF7 cells
microarray: suitable
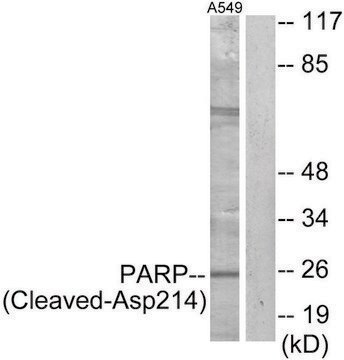
western blot: 1:200 using MCF7 human mammary adenocarcinoma cell extract
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... PARP1(142)
General description
Specificity
Immunogen
Application
Biochem/physiol Actions
Physical form
Disclaimer
Ještě jste nenalezli správný produkt?
Vyzkoušejte náš produkt Nástroj pro výběr produktů.
recommended
Storage Class
10 - Combustible liquids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.