A1075
Amyloid β Protein Fragment 1-40
≥90% (HPLC), powder
Synonyma:
Aβ40
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Empirický vzorec (Hillův zápis):
C194H295N53O58S
Číslo CAS:
Molekulová hmotnost:
4329.80
MDL number:
UNSPSC Code:
12352202
NACRES:
NA.32
Doporučené produkty
Quality Level
assay
≥90% (HPLC)
form
powder
color
white
solubility
1% acetic acid: 1 mg/mL
saline: insoluble
UniProt accession no.
storage temp.
−20°C
Gene Information
human ... APP(351)
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
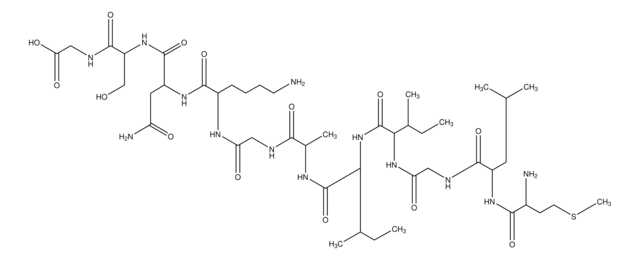
Amino Acid Sequence
Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-Tyr-Glu-Val-His-His-Gln-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Val-Gly-Ser-Asn-Lys-Gly-Ala-Ile-Ile-Gly-Leu-Met-Val-Gly-Gly-Val-Val
General description
Amyloid β Protein Fragment 1-40 (Aβ40) is derived from the amyloid-β protein (Aβ), which is mapped to human chromosome 21q21.3. Aβ40 is predominantly present in the vascular amyloid deposits. Aβ40 comprises of C-terminal membrane insertion domain. It shows structural transition from random coil to a α-helical structure in a water-micelle medium.
Application
Amyloid β Protein Fragment 1-40 has been used:
- in the temperature based conformational studies using Fourier transform infrared/differential scanning calorimetry (FT-IR/DSC) studies
- as a reference standard in sandwich-type enzyme immunoassay for quantifying amyloid A4 protein in cerebrospinal fluid of patients with head trauma
- as a component of embryonic stem cell medium to inhibit amyloid deposition in fibroblasts
Biochem/physiol Actions
Amyloid β Protein Fragment 1-40 (Aβ40) forms cation based ion channels.
Amyloid β-protein is neurotrophic and neurotoxic in vivo and in vitro in human and rat neuronal cell cultures. β-Amyloid peptides (amino acids 1-42 and 1-43) are the major constituents of senile plaques and neurofibrillary tangles that occur in the hippocampus, neocortex, and amygdala of patients with Alzheimer′s disease.
Reconstitution
For maximal biological activity, dilute the stock in calcium-free PBS to 1 mg/ml and incubate at 37 °C for 4 days.
Other Notes
Lyophilized from 0.1% TFA in H2O
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Lot/Batch Number
Nevidíte správnou verzi?
Potřebujete-li konkrétní verzi, můžete vyhledat daný certifikát podle čísla dávky nebo čísla šarže.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Amyloid beta protein 1-40 and 1-42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease
Mehta PD, et al.
Neuroscience Letters, 304(1-2), 102-106 (2001)
K Ueda et al.
Brain research, 639(2), 240-244 (1994-03-14)
The neurotoxic effects of soluble and aggregated synthetic amyloid beta protein (A beta P) have been investigated in rat primary cultures. Freshly solubilized beta(1-40) was neurotoxic not to immature, but to mature hippocampal neurons. On the other hand, aggregated beta(1-40)
Sandwich-Type Enzyme Immunoassay for Amyloid A4 Protein in Cerebrospinal Fluid From Patients with Head Trauma
PIRIM I
Turkish Journal of Medical Sciences, 31(1), 47-50 (2001)
Charlotte E Teunissen et al.
Journal of Alzheimer's disease : JAD, 62(4), 1857-1863 (2018-04-05)
The 42 amino acid form of amyloid-β (Aβ42) plays a key role in the pathogenesis of Alzheimer's disease (AD) and is a core biomarker for the diagnosis of AD. Numerous studies have shown that cerebrospinal fluid (CSF) Aβ42 concentrations are
Temperature-induced conformational changes in amyloid beta (1-40) peptide investigated by simultaneous FT-IR microspectroscopy with thermal system
Chu HL and Lin SY
Biophysical Chemistry, 89(2-3), 173-180 (2001)
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.







![[Gln22]-Amyloid β 1-40 human ≥95% (HPLC)](/deepweb/assets/sigmaaldrich/product/images/707/874/59f84b84-17c2-494f-b2ae-4ca860b83976/640/59f84b84-17c2-494f-b2ae-4ca860b83976.jpg)