Y0000529
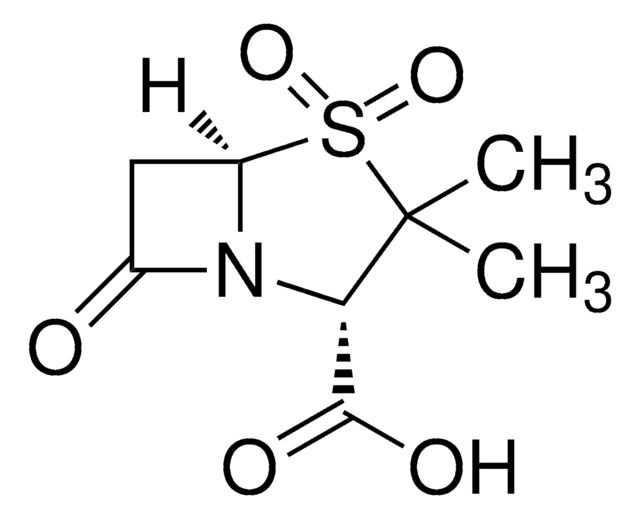
Sulbactam sodium
European Pharmacopoeia (EP) Reference Standard
Synonyma:
Sulbactam sodium salt
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Doporučené produkty
grade
pharmaceutical primary standard
API family
sulbactam
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C8H11NO5S.Na/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14;/h5-6H,3H2,1-2H3,(H,11,12);/q;+1/p-1/t5-,6+;/m1./s1
InChI key
NKZMPZCWBSWAOX-IBTYICNHSA-M
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Sulbactam sodium EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Č. produktu
Popis
Stanovení ceny
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Lot/Batch Number
Je nám líto, ale pro tento produkt momentálně nemáme COA k dispozici online.
Potřebujete-li pomoc, obraťte se na Zákaznická podpora
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
In vitro susceptibilities of clinical isolates of Escherichia coli and Klebsiella species to CSE1034 and other β-lactams.
Manu Chaudhary et al.
The Journal of antibiotics, 66(8), 495-497 (2013-04-25)
Sutep Jaruratanasirikul et al.
Antimicrobial agents and chemotherapy, 57(7), 3441-3444 (2013-05-08)
The aim of this study was to reveal population pharmacokinetics and assess the efficacies of various dosage regimens of sulbactam in terms of the probability of target attainment with this agent over a range of MICs. Monte Carlo simulations were
Seyedali Seyedmajidi et al.
Arab journal of gastroenterology : the official publication of the Pan-Arab Association of Gastroenterology, 14(1), 1-5 (2013-04-30)
Selection of the best drug regimens for eradication of Helicobacter pylori infection especially in patients at risk of peptic ulcer relapses and the development of complications is challenging. This study assessed and compared the efficacy of the two common PPI
Mengtao Zhou et al.
Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.], 13(3), 212-215 (2013-05-31)
Our aim was to investigate the efficiency of continuous regional intra-arterial infusion (CRAI) with antisecretory agents and antibiotics in the treatment of infected pancreatic necrosis. CRAI was used as a new clinical technique to treat acute pancreatitis patients during a
Maura S de Oliveira et al.
Clinics (Sao Paulo, Brazil), 68(4), 569-573 (2013-06-20)
The objective of this study was to evaluate whether the outcomes of carbapenem-resistant Acinetobacter infections treated with ampicillin/sulbactam were associated with the in vitro susceptibility profiles. Twenty-two infections were treated with ampicillin/sulbactam. The median treatment duration was 14 days (range:
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.