PHR1336
Nimesulide
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyma:
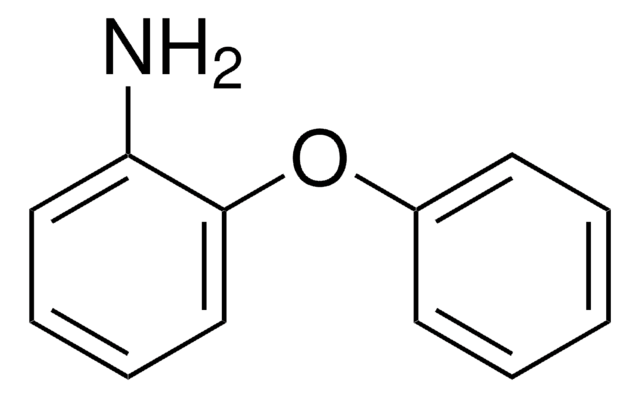
Nimesulide, N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide
About This Item
Doporučené produkty
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur. N0845000
API family
nimesulide
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
CS(NC1=C(OC2=CC=CC=C2)C=C([N+]([O-])=O)C=C1)(=O)=O
InChI
1S/C13H12N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h2-9,14H,1H3
InChI key
HYWYRSMBCFDLJT-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Nimesulide is a selective cyclooxygenase-2 (COX-2) inhibitor that can potentially be used as a therapeutic agent for the treatment of inflammatory conditions. It is also a non-steroidal anti-inflammatory drug (NSAID) that possesses analgesic and antipyretic characters.
Application
Biochem/physiol Actions
Analysis Note
Other Notes
Footnote
Recommended products
signalword
Danger
hcodes
pcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.