P2698000
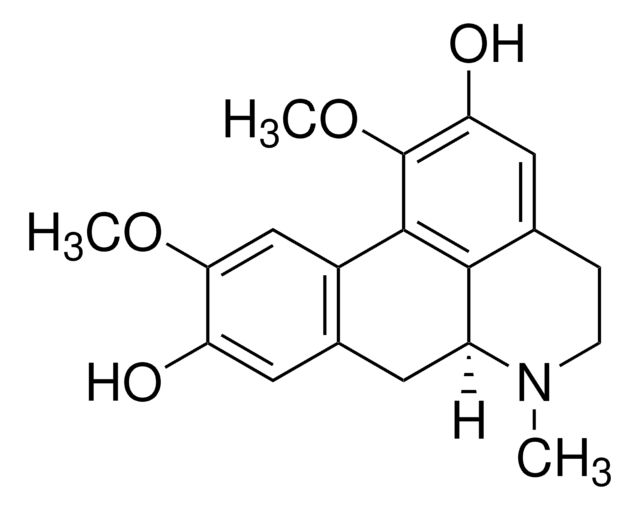
Prednicarbate
European Pharmacopoeia (EP) Reference Standard
Synonyma:
(11β)-17-[(Ethoxycarbonyl)oxy]-11-hydroxy-21-(1-oxopropoxy)pregna-1,4-diene-3,20-dione
About This Item
Doporučené produkty
grade
pharmaceutical primary standard
API family
prednicarbate
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
InChI
1S/C27H36O8/c1-5-22(31)34-15-21(30)27(35-24(32)33-6-2)12-10-19-18-8-7-16-13-17(28)9-11-25(16,3)23(18)20(29)14-26(19,27)4/h9,11,13,18-20,23,29H,5-8,10,12,14-15H2,1-4H3
Inchi Key
FNPXMHRZILFCKX-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
Packaging
Other Notes
related product
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Je nám líto, ale pro tento produkt momentálně nemáme COA k dispozici online.
Potřebujete-li pomoc, obraťte se na Zákaznická podpora
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.