E6376
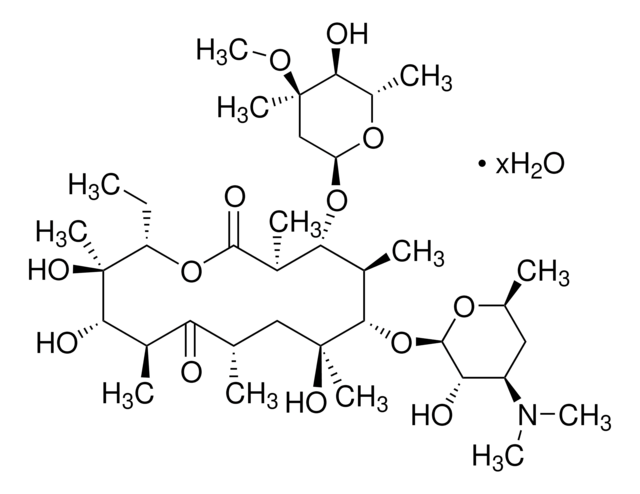
Erythromycin
potency: ≥850 μg per mg
Synonyma:
Erythromycin A
About This Item
Doporučené produkty
biological source
Streptomyces erythreus
Quality Level
form
powder
potency
≥850 μg per mg
color
white
solubility
ethanol: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
protein synthesis | interferes
SMILES string
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChI key
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Gene Information
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
Erythromycin is an antibiotic produced by growth of certain strains of Streptomyces erythreus. This product is composed largely of erythromycin A with small amounts of erythromycins B and C and is recommended for concentration at 100 mg/L. Concentrations between 50 and 200 mg/L have also proven effective in controlling bacterial growth. Erythromycin has been used as a motilin receptor agonist, to block respiratory glycoconjugate secretion in human airways in vitro, and for selecting plasmid-cured and recombinant lactococcus lactis MG1363 strains.
Biochem/physiol Actions
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Caution
Preparation Note
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Sortimentní položky
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.