MABF39-I
Anti-DC-STAMP Antibody, clone 1A2
clone 1A2, from mouse
Synonyma:
Dendritic cell-specific transmembrane protein, DC-STAMP, Dendrocyte-expressed seven transmembrane protein, FIND, hDC-STAMP, IL-four-induced protein, Transmembrane 7 superfamily member 4
About This Item
Doporučené produkty
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
1A2, monoclonal
species reactivity
mouse, human
technique(s)
flow cytometry: suitable
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
isotype
IgG2aκ
NCBI accession no.
UniProt accession no.
target post-translational modification
unmodified
Gene Information
human ... DCSTAMP (81501)
Související kategorie
General description
Specificity
Immunogen
Application
Inflammation & Immunology
Osteobiology
Western Blotting Analysis: 1.0 µg/mL from a representative lot detected DC-STAMP in thymus and spleen lysates from wild-type mice, and greatly reduced DC-STAMP in thymus and spleen lysates from Dcstamp-knockout mice (Courtesy of Grace Chiu, Ph.D., University of Rochester Medical Center, NY, USA).
Western Blotting Analysis: A representative lot detected the ~106 kDa dimeric and the ~53 kDa monomeric DC-STAMP band by Western blotting under non-denatured and denatured condition, respectively, following DC-STAMP immunoprecipitation using murine RAW 264.7 macrophage lysate (Mensah, K.A., et al. (2010). J. Cell. Physiol. 223(1):76-83).
Flow Cytometry Analysis: A representative lot detected DC-STAMP-positive lymphoctes and monocytes in purified human PBMCs (Chiu, Y.H., et al. (2012). J. Bone Miner. Res. 27(1):79-92).
Flow Cytometry Analysis: A representative lot detected DC-STAMP surface expression on murine RAW 264.7 macrophages and murine bone marrow-derived CD11b+ monocytes. DC-STAMP is expressed on osteoclast precursor (OCP) cells as a dimer, which is efficiently detected by flow cytometry using clone 1A2 (Mensah, K.A., et al. (2010). J. Cell. Physiol. 223(1):76-83).
Function Analysis: A representative lot inhibited RANKL & M-CSF treatment-induced osteoclasts (OC) formation in human PBMCs & monocytes cultures (Chiu, Y.H., et al. (2012). J. Bone Miner. Res. 27(1):79-92).
Function Analysis: A representative lot inhibited RANKL treatment-induced osteoclasts (OC) formation in RAW 264.7 and murine bone marrow macrophages cultures (Mensah, K.A., et al. (2010). J. Cell. Physiol. 223(1):76-83).
Immunohistochemistry Analysis: A representative lot detected DC-STAMP expressionon in multinucleated ‘osteoclast-like’ giant cells in human giant cell tumor of bone (Chiu, Y.H., et al. (2012). J. Bone Miner. Res. 27(1):79-92).
Immunocytochemistry Analysis: A representative lot detected DC-STAMP-positive human PBMCs using 10% NBF-fixed, paraffin-embedded cell preparation (Chiu, Y.H., et al. (2012). J. Bone Miner. Res. 27(1):79-92).
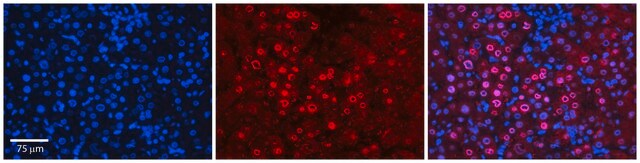
Immunocytochemistry Analysis: A representative lot detected differential DC-STAMP intracellular localizations by fluorescent immunocytochemistry staining of 4% paraformaldehyde-fixed, 0.1% saponin-permeabilized murine bone marrow macrophages at different time points during osteoclastogenesis upon RANKL treatment (Mensah, K.A., et al. (2010). J. Cell. Physiol. 223(1):76-83).
Immunoprecipitation Analysis: A representative lot immunoprecipitated DC-STAMP from the lysates of human monocytes (Chiu, Y.H., et al. (2012). J. Bone Miner. Res. 27(1):79-92).
Immunoprecipitation Analysis: A representative lot immunoprecipitated DC-STAMP from the membrane extracts of murine RAW 264.7 macrophages (Mensah, K.A., et al. (2010). J. Cell. Physiol. 223(1):76-83).
Quality
Western Blotting Analysis: 4.0 µg/mL of this antibody detected DC-STAMP in 10 µg of mouse thymus lysate.
Target description
Physical form
Storage and Stability
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
Other Notes
Disclaimer
Ještě jste nenalezli správný produkt?
Vyzkoušejte náš produkt Nástroj pro výběr produktů.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.