05-414
Anti-CrkL Antibody, clone 5-6
clone 5-6, Upstate®, from mouse
About This Item
Doporučené produkty
biological source
mouse
Quality Level
antibody form
purified antibody
antibody product type
primary antibodies
clone
5-6, monoclonal
species reactivity
mouse, rat, human
manufacturer/tradename
Upstate®
technique(s)
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
isotype
IgG2aκ
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... CRKL(1399)
General description
The antibody does not cross-react with CRKI or CRKII.
Specificity
Immunogen
Application
Signaling
Cytoskeletal Signaling
Quality
Western Blot Analysis: Antibody detected CRKL in 15 μg of K562 cell lysate.
Target description
Physical form
Storage and Stability
Analysis Note
Western Blot and Immunoprecipitation:
K562 cell lysate
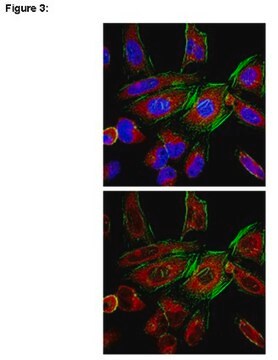
Immunocytochemistry:
Hela cells
Legal Information
Disclaimer
Ještě jste nenalezli správný produkt?
Vyzkoušejte náš produkt Nástroj pro výběr produktů.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.