About This Item
Lineární vzorec:
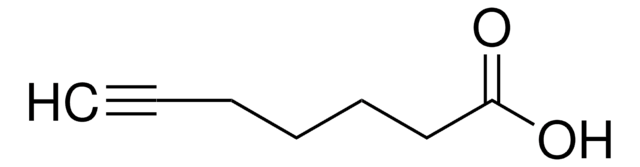
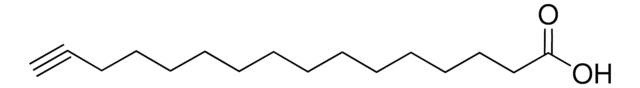
HC≡C(CH2)8CO2H
Číslo CAS:
Molekulová hmotnost:
182.26
Beilstein/REAXYS Number:
1704918
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
Quality Level
assay
95%
bp
180 °C/15 mmHg (lit.)
mp
40-42 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)CCCCCCCCC#C
InChI
1S/C11H18O2/c1-2-3-4-5-6-7-8-9-10-11(12)13/h1H,3-10H2,(H,12,13)
InChI key
OAOUTNMJEFWJPO-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
10-Undecynoic acid (10- UDYA, UDY) is an acetylenic fatty acid. It is reported as highly selective irreversible inhibitor of hepatic ω- and ω-1-lauric acid hydroxylases. Enzyme catalyzed esterification of 10-undecynoic acid has been reported. UDY has been reported to be synthesized by the dehydrobromination of 10-undecenoic acid.
Application
10-Undecynoic acid was employed as model compound to investigate the microwave assisted surface click reactions catalyzed with Cu(II)/sodium L-ascorbate.†
It may be used:
It may be used:
- As a biochemical probe in an assay for the microsomal hydroxylation of lauric acid (LA), based on HPLC with flow-through radiochemical detection.
- To form molecular layers by adsorbing on the fluorite surface.
- In the supercritical hydrothermal synthesis of iron oxide nanoparticles.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
P R Ortiz de Montellano et al.
The Journal of biological chemistry, 259(7), 4136-4141 (1984-04-10)
The terminal acetylenic analogue of lauric acid, 11-dodecynoic acid (11-DDYA), specifically inactivates hepatic cytochrome P-450 enzymes that catalyze omega- and omega-1-hydroxylation of lauric acid. The inactivation, as required for a suicidal process, is NADPH- and time-dependent and follows pseudo-first order
Studies of lipase-catalyzed esterification reactions of some acetylenic fatty acids.
Jie MSFLK and Xun F.
Lipids, 33(1), 71-75 (1998)
Formation and wetting characteristics of adsorbed layers of unsaturated carboxylic acids at a fluorite surface.
Drelich J, et al.
Journal of Colloid and Interface Science, 178(2), 720-732 (1996)
R C Zangar et al.
Archives of biochemistry and biophysics, 337(2), 217-224 (1997-01-15)
CYP2B, CYP4A, and CYP2E1 mRNA levels are elevated in response to pathophysiological conditions, such as diabetes, high-fat diet, and fasting, in which lipids and ketone bodies are increased. In order to avoid confounding hormonal effects, we utilized primary rat hepatocytes
M C Romano et al.
Analytical biochemistry, 170(1), 83-93 (1988-04-01)
An assay for the microsomal hydroxylation of lauric acid (LA), based on HPLC with flow-through radiochemical detection, has been developed. Conditions were optimized for resolution and quantitation of three microsomal metabolites of LA, one of which has not been reported
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.