302589
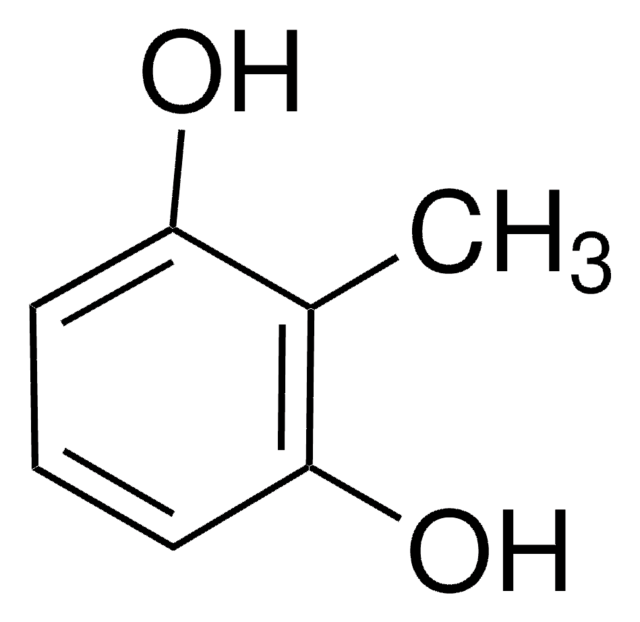
2-Methylresorcinol
98%
Synonym(s):
2,6-Dihydroxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3C6H3(OH)2
CAS Number:
Molecular Weight:
124.14
Beilstein:
2042177
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
264 °C (lit.)
mp
114-120 °C (lit.)
SMILES string
Cc1c(O)cccc1O
InChI
1S/C7H8O2/c1-5-6(8)3-2-4-7(5)9/h2-4,8-9H,1H3
InChI key
ZTMADXFOCUXMJE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The reaction between 2-methylresorcinol and 2-alkenals was studied to investigate the scavenging ability of m-diphenols for the 2-alkenals formed during lipid oxidation.
Application
2-Methylresorcinol was used in the synthesis of:
- C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene
- tripyrrane analogs
- series of novel aromatic benziporphyrins
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Eye Dam. 1 - Skin Sens. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
275.0 °F - closed cup
Flash Point(C)
135 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Francisco J Hidalgo et al.
Food chemistry, 160, 118-126 (2014-05-07)
The reaction between m-diphenols (resorcinol, 2-methylresorcinol, 2,5-dimethylresorcinol, 3-methylphenol, orcinol, and phloroglucinol) and 2-alkenals (2-pentenal and 2-octenal) was studied in an attempt to understand the chemical pathways involved in the scavenging ability of m-diphenols for the 2-alkenals produced as a consequence
Timothy D Lash et al.
The Journal of organic chemistry, 76(15), 6295-6308 (2011-06-23)
Tripyrrane analogues were prepared by reacting resorcinol or 2-methylresorcinol with 2 equiv of an acetoxymethylpyrrole in the presence of p-toluenesulfonic acid and calcium chloride. Following removal of the benzyl ester protective groups, the resorcinol-derived benzitripyrrane was reacted with a pyrrole
Hamza M Abosadiya et al.
Molecules (Basel, Switzerland), 18(11), 13369-13384 (2013-11-01)
C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene (I) was synthesized by cyclocondensation of 5-bromo-2-hydroxybenzaldehyde and 2-methylresorcinol in the presence of concentrated HCl. Compound I was characterized by infrared and nuclear magnetic resonance spectroscopic data. X-ray analysis showed that this compound crystallized in a triclinic system with
Kae Miyake et al.
Chemical communications (Cambridge, England), (2)(2), 178-179 (2004-01-23)
Acid catalyzed condensation of resorcinol or 2-methylresorcinol with 2 equiv. of an acetoxymethylpyrrole gave bis(pyrrolylmethyl)benzene derivatives in moderate yields; these afforded a series of novel aromatic benziporphyrins using the MacDonald "3 + 1" methodology.
C Naumann et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 7(8), 1637-1645 (2001-05-15)
The preparation of cavitands composed of 4, 5, 6, and 7 aromatic subunits ([n]cavitands, n=4-7) is described. The simple, two-step synthetic procedure utilized readily available starting materials (2-methylresorcinol and diethoxymethane). The two cavitand products having 4 and 5 aromatic subunits
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service