144894
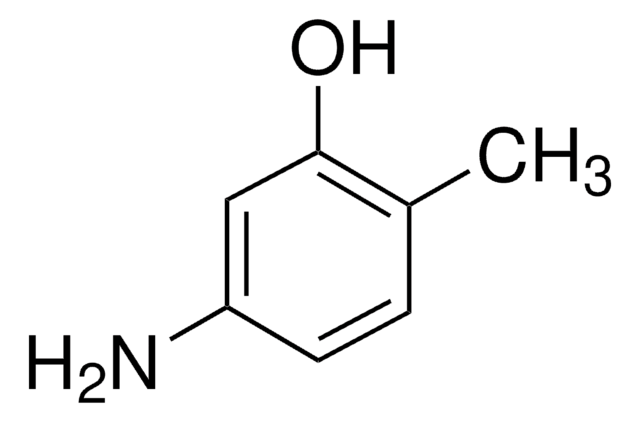
4-Amino-3-methylphenol
97%
Synonym(s):
2-Amino-5-hydroxytoluene, 4-Amino-m-cresol, 4-Hydroxy-2-methylaniline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H3(CH3)OH
CAS Number:
Molecular Weight:
123.15
Beilstein:
2078803
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
176-179 °C (lit.)
SMILES string
Cc1cc(O)ccc1N
InChI
1S/C7H9NO/c1-5-4-6(9)2-3-7(5)8/h2-4,9H,8H2,1H3
InChI key
QGNGOGOOPUYKMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Amino-3-methylphenol is a metabolite of 3-methyl-4-nitrophenol. It is a major metabolite of carcinogenic o-toluidine and causes DNA damage in the presence of Cu(II).
Application
4-Amino-3-methylphenol was used in synthesis of a new type of tweezer-molecule in which a strongly preferred binding conformation is generated by convergent, intramolecular hydrogen bonding.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y Ohkuma et al.
Archives of biochemistry and biophysics, 372(1), 97-106 (1999-11-24)
Mechanisms of DNA damage by metabolites of carcinogenic o-toluidine in the presence of metals were investigated by the DNA sequencing technique using (32)P-labeled human DNA fragments. 4-Amino-3-methylphenol, a major metabolite, caused DNA damage in the presence of Cu(II). Predominant cleavage
Robert A Kanaly et al.
Journal of agricultural and food chemistry, 53(16), 6426-6431 (2005-08-04)
Biotransformation of the environmental pollutant 3-methyl-4-nitrophenol (MNP), a newly characterized estrogenic chemical, and the primary breakdown product of the heavily used insecticide fenitrothion was investigated using a common soil fungus. In 96 h, daily culture sacrifice, extraction, and analysis showed
Howard M Colquhoun et al.
Faraday discussions, 143, 205-220 (2009-01-01)
A novel type of tweezer molecule containing electron-rich 2-pyrenyloxy arms has been designed to exploit intramolecular hydrogen bonding in stabilising a preferred conformation for supramolecular complexation to complementary sequences in aromatic copolyimides. This tweezer-conformation is demonstrated by single-crystal X-ray analyses
Emel Ermiş et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 243, 118761-118761 (2020-08-28)
Eight new azomethine compounds (3a-3h) containing thiophene and aminophenol functionality were synthesized in excellent yields by using conventional heating and microwave assisted synthesis methods. The structures of newly synthesized compounds were characterized by spectroscopic techniques such as UV-Vis, FTIR, 1H
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service