15256
BSA+TMCS
for GC derivatization, LiChropur™, 93.0-97.0% (GC)
Sinónimos:
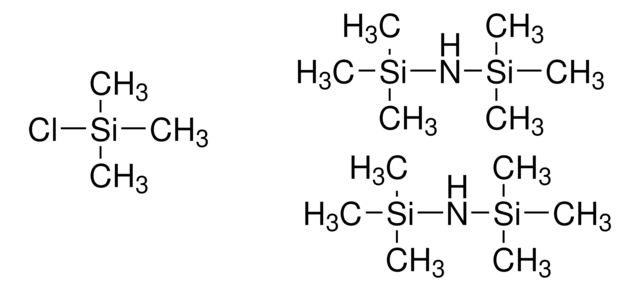
Bis(trimethylsilyl)acetamide + Trimethylchlorosilane
About This Item
Productos recomendados
grade
for GC derivatization
Quality Level
assay
93.0-97.0% (GC)
quality
LiChropur™
composition
trimethylchlorosilane (minor component), 3.0-5.0% GC
reaction suitability
reagent type: derivatization reagent
reaction type: Silylations
technique(s)
gas chromatography (GC): suitable
General description
Application
Features and Benefits
- BSA+TMCS has good solvent properties and can function as a silylation reagent without additional solvents.
- Alternatively, the mixture is very soluble in most commonly used silylation solvents.
- This combination is extremely sensitive to moisture and should be handled under dry conditions.
Other Notes
Legal Information
Related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1A
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
53.6 °F - closed cup
flash_point_c
12 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico