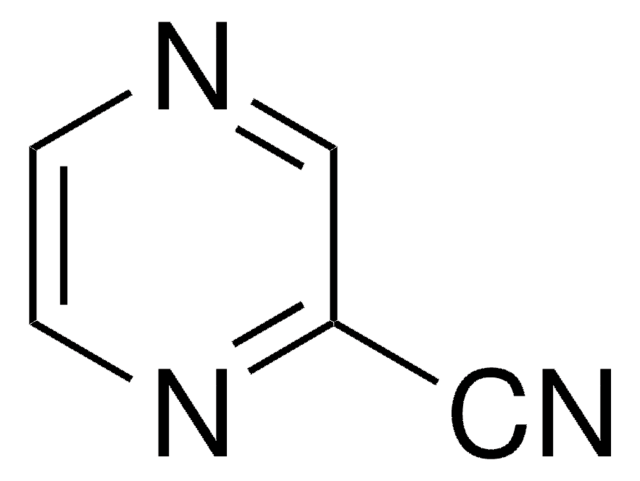
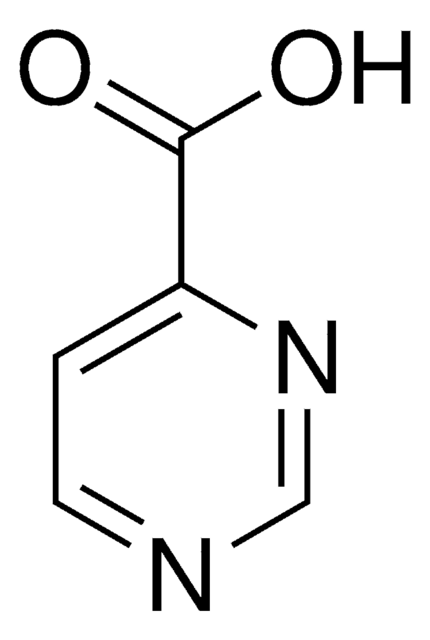
P56100
Pyrazinecarboxylic acid
99%
Sinónimos:
Pyrazinoic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H4N2O2
Número de CAS:
Peso molecular:
124.10
Beilstein:
112305
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
99%
Formulario
powder
mp
222-225 °C (dec.) (lit.)
cadena SMILES
OC(=O)c1cnccn1
InChI
1S/C5H4N2O2/c8-5(9)4-3-6-1-2-7-4/h1-3H,(H,8,9)
Clave InChI
NIPZZXUFJPQHNH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Mohamed Abdel-Aziz et al.
European journal of medicinal chemistry, 45(8), 3384-3388 (2010-05-22)
A series of pyrazine-2-carboxylic acid hydrazide derivatives were synthesized and screened for their activity against Mycobacterium tuberculosis. The results show that pyrazine-2-carboxylic acid hydrazide-hydrazone derivatives 3a-l were less active than pyrazinamide. In contrast, the N(4)-ethyl-N(1)-pyrazinoyl-thiosemicarbazide 4 showed the highest activity
Ping Lu et al.
Antimicrobial agents and chemotherapy, 55(11), 5354-5357 (2011-08-31)
Pyrazinoic acid, the active form of the first-line antituberculosis drug pyrazinamide, decreased the proton motive force and respiratory ATP synthesis rates in subcellular mycobacterial membrane assays. Pyrazinoic acid also significantly lowered cellular ATP levels in Mycobacterium bovis BCG. These results
Stefania Tanase et al.
Dalton transactions (Cambridge, England : 2003), (15)(15), 2026-2033 (2008-04-03)
The reactivity towards H(2)O(2) of the complexes [Fe(pca)(2)(py)(2)].py (1) and Na(2){[Fe(pca(3))](2)O}.2H(2)O.CH(3)CN (2) (where pca(-) is pyrazine-2-carboxylate) and their catalytic activity in the oxidation of hydrocarbons is reported. Addition of H(2)O(2) to 1 results in the formation of a dinuclear Fe(III)-(mu-O)-Fe(III)
Mirko Zimic et al.
Tuberculosis (Edinburgh, Scotland), 92(1), 84-91 (2011-10-19)
Pyrazinamide is one of the most important drugs in the treatment of latent Mycobacterium tuberculosis infection. The emergence of strains resistant to pyrazinamide represents an important public health problem, as both first- and second-line treatment regimens include pyrazinamide. The accepted
Rustam Z Khaliullin et al.
The journal of physical chemistry. B, 109(38), 17984-17992 (2006-07-21)
Experimental studies by Shul'pin and co-workers have shown that vanadate anions in combination with pyrazine-2-carboxylic acid (PCA identical with pcaH) produce an exceptionally active complex that promotes the oxidation of alkanes and other organic molecules. Reaction of this complex with
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico