667005
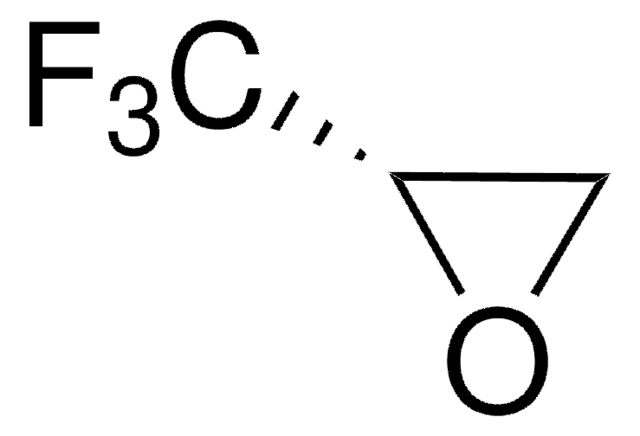
(R)-(+)-3,3,3-Trifluoro-1,2-epoxypropane
97%
Sinónimos:
(R)-(+)-2-(Trifluoromethyl)oxirane
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C3H3F3O
Número de CAS:
Peso molecular:
112.05
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
refractive index
n20/D <1.300
bp
25-32 °C
density
1.294 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
FC(F)(F)[C@H]1CO1
InChI
1S/C3H3F3O/c4-3(5,6)2-1-7-2/h2H,1H2/t2-/m1/s1
InChI key
AQZRARFZZMGLHL-UWTATZPHSA-N
Categorías relacionadas
Application
(R)-(+)-3,3,3-Trifluoro-1,2-epoxypropane can be used as a substrate to synthesize:
- Substituted trifluoro amino propanols, which are found to be potent inhibitors of cholesteryl ester transfer protein.
- (2R) Trifluoro-(methoxybenzyloxy)-propanol (chiral glycol) by reacting with 4-methoxybenzyl alcohol in the presence of NaH. Chiral glycol intermediate is further utilized for the preparation of trifluoromethyl glycol carbamates as potential monoacylglycerol lipase (MAGL) inhibitors.
signalword
Danger
hcodes
pcodes
Hazard Classifications
Flam. Liq. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-14.8 °F
flash_point_c
-26 °C
ppe
Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Discovery of trifluoromethyl glycol carbamates as potent and selective covalent monoacylglycerol lipase (MAGL) inhibitors for treatment of neuroinflammation
McAllister LA, et al.
Journal of Medicinal Chemistry, 61(7), 3008-3026 (2018)
Discovery of a simple picomolar inhibitor of cholesteryl ester transfer protein
Reinhard EJ, et al.
Journal of Medicinal Chemistry, 46(11), 2152-2168 (2003)
Emily J Reinhard et al.
Journal of medicinal chemistry, 46(11), 2152-2168 (2003-05-16)
A novel series of substituted N-[3-(1,1,2,2-tetrafluoroethoxy)benzyl]-N-(3-phenoxyphenyl)-trifluoro-3-amino-2-propanols is described which potently and reversibly inhibit cholesteryl ester transfer protein (CETP). Starting from the initial lead 1, various substituents were introduced into the 3-phenoxyaniline group to optimize the relative activity for inhibition of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico