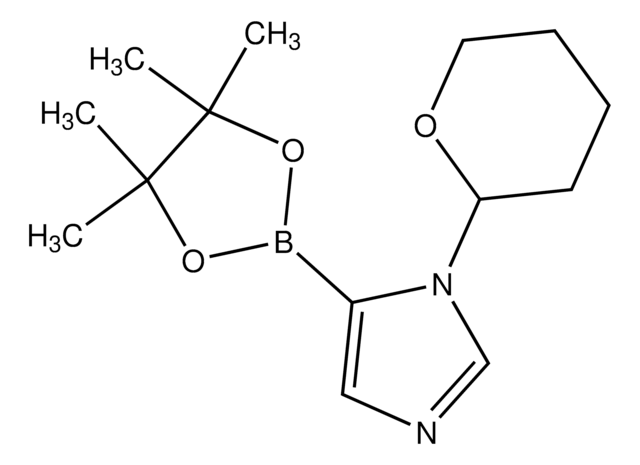
632732
1-Boc-pyrazole-4-boronic acid pinacol ester
97%
Sinónimos:
1,1-dimethylethyl ester 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-Pyrazole-1-carboxylic acid, 1-tert-Butoxycarbonyl-4-(4,4,5,5-tetramethyl[1,3,2]dioxaborolan-2-yl)pyrazole, 1-Boc-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole, 1-Boc-4-pyrazoleboronic acid pinacol ester, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-Boc-pyrazole, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole-1-carboxylic acid tert-butyl ester, tert-Butyl 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-pyrazolecarboxylate, [1-(tert-Butoxycarbonyl)-1H-pyrazol-4-yl]boronic acid pinacol ester
About This Item
Productos recomendados
Quality Level
assay
97%
form
solid
mp
82-86 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)(C)OC(=O)n1cc(cn1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C14H23BN2O4/c1-12(2,3)19-11(18)17-9-10(8-16-17)15-20-13(4,5)14(6,7)21-15/h8-9H,1-7H3
InChI key
IPISOFJLWYBCAV-UHFFFAOYSA-N
Application
- Suzuki Coupling
- Copper-catalyzed azidation
Reagent used in Preparation of
- Selective quinazolinyl-phenol inhibitors of CHK1 as potential antitumors and radioprotectants
- Stereoselective synthesis of selective Cathepsin inhibitors
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)