539112
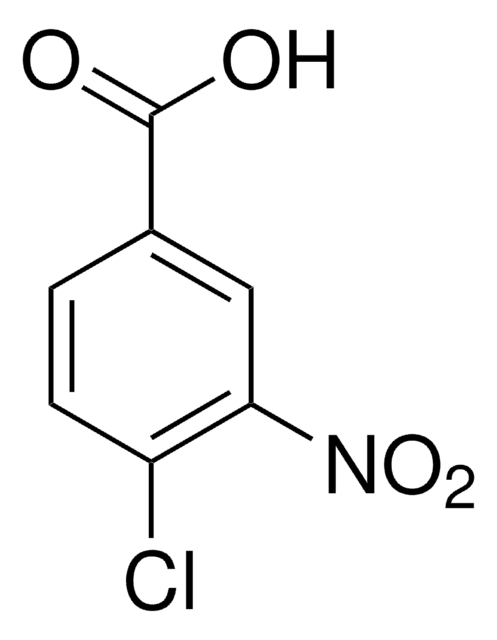
4-Bromo-1-fluoro-2-nitrobenzene
96%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
BrC6H3(F)NO2
Número de CAS:
Peso molecular:
220.00
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
96%
índice de refracción
n20/D 1.575 (lit.)
bp
240-241 °C (lit.)
mp
18-19 °C (lit.)
densidad
1.786 g/mL at 25 °C (lit.)
grupo funcional
bromo
fluoro
nitro
cadena SMILES
[O-][N+](=O)c1cc(Br)ccc1F
InChI
1S/C6H3BrFNO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
Clave InChI
UQEANKGXXSENNF-UHFFFAOYSA-N
Descripción general
4-Bromo-1-fluoro-2-nitrobenzene undergoes Sonogashira reaction with 2-fluoronitrobenzene to afford predominantly the bromo displacement product.
Aplicación
4-Bromo-1-fluoro-2-nitrobenzene may be used in the synthesis of:
- 6-bromo-1H-benzo[d][1,2,3]triazol-1-ol
- 2-(4-bromo-2-nitrophenylamino)-5-methylthiophene-3-carbonitrile
- dibenzoxazepine analog, as potent sodium channel blocker
- 4-(4-bromo-2-nitrophenyl)piperazine-1-carboxylic acid tert-butylester
Used in the synthesis of anti-inflammatory agents.
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Katie M Lutker et al.
Crystal growth & design, 8(1), 136-139 (2008-01-01)
Bis(5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrilyl)acetylene, a derivative of the highly polymorphic compound 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY) that possesses two chromophores electronically coupled through a triple bond, was found to be trimorphic. Structural data for two of these forms indicates that symmetry is maintained in one structure
Patrick L DeRoy et al.
Organic letters, 9(14), 2741-2743 (2007-06-08)
The nucleophilic aromatic substitution reaction between electron-deficient aryl fluorides and terminal alkynes is shown to be efficiently promoted by sodium bis(trimethylsilyl)amide as a base. Moderate to excellent yields of 2-ethynylnitrobenzene products can be obtained under mild conditions.
Venkateshwarlu Gurram et al.
Advanced synthesis & catalysis, 357(2-3), 451-462 (2015-03-03)
Benzotriazoles are a highly important class of compounds with broad-ranging applications in such diverse areas as medicinal chemistry, as auxiliaries in organic synthesis, in metallurgical applications, in aircraft deicing and brake fluids, and as antifog agents in photography. Although there
Tomoki Kawai et al.
Nuclear medicine and biology, 40(5), 705-709 (2013-05-28)
As a first trial for in vivo imaging of β-secretase (BACE1) in Alzheimer's disease brain, we applied a novel non-peptidergic small molecule which has high affinity to the enzyme, naphthalene-1-carboxylic acid (3'-chloro-4'-fluoro-4-piperazin-1-yl-biphenyl-3-yl)amide (NCFB) into positron emission tomography (PET) probe. In
Erik Rytter Ottosen et al.
Journal of medicinal chemistry, 46(26), 5651-5662 (2003-12-12)
We wish to report the synthesis and structure-activity relationship (SAR) of a series of 4-aminobenzophenones, as a novel compound class with high antiinflammatory activity. Our initial lead, (4-[(2-aminophenyl)amino]phenyl)(phenyl)methanone (3), was systematically optimized and resulted in compounds that potently inhibited the
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)