528994
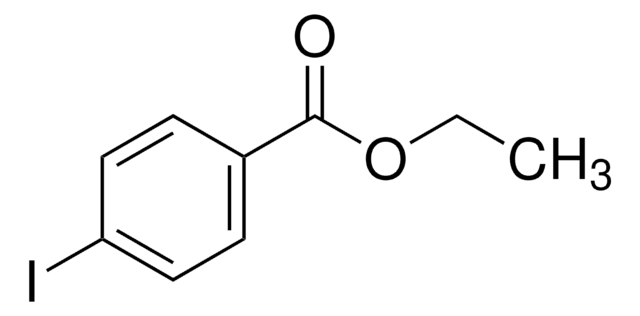
Ethyl 3-iodobenzoate
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
IC6H4CO2C2H5
Número de CAS:
Peso molecular:
276.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
refractive index
n20/D 1.581 (lit.)
bp
272 °C (lit.)
density
1.64 g/mL at 25 °C (lit.)
functional group
ester
iodo
SMILES string
CCOC(=O)c1cccc(I)c1
InChI
1S/C9H9IO2/c1-2-12-9(11)7-4-3-5-8(10)6-7/h3-6H,2H2,1H3
InChI key
POGCXCWRMMXDAQ-UHFFFAOYSA-N
General description
Ethyl 3-iodobenzoate is a halogenated aromatic ester. It affords arylzinc bromide via reaction with i-PrMgBr in THF, followed by reaction with ZnBr2.
Application
Ethyl 3-iodobenzoate may be used to synthesize:
- arylzinc bromide
- functionalized arylmagnesium compound
- ethyl3-phenylbenzoate
- ethyl 3-[(12-tert-butyldimethylsilyloxymethyl-1,12-dicarba-closo-dodecaboran)-1-yl]benzoate
- ethyl 3-(4-methoxy-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)benzoate
- ethyl 3-(1-methyl-2-oxo-4-phenyl-1,2-dihydroquinolin-3-yl)benzoate
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Copper catalyzed conjugate addition of highly functionalized arylmagnesium compounds to enones.
Varchi G, et al.
Tetrahedron, 56(18), 2727-2731 (2000)
Ni (II)-catalyzed cross-coupling between polyfunctional arylzinc derivatives and primary alkyl iodides.
Giovannini R and Knochel P.
Journal of the American Chemical Society, 120(43), 11186-11187 (1998)
Shinya Fujii et al.
Bioorganic & medicinal chemistry, 17(1), 344-350 (2008-11-22)
A novel series of androgen receptor (AR) ligands bearing an acidic heterocycle with hydrogen-bonding ability as the terminal polar group was developed. Since most non-steroidal AR ligands so far known are structurally limited to nitro- or cyanobenzanilide as the polar
Synthesis of 3, 4-Disubstituted Quinolin-2-(1H)-ones via Palladium-Catalyzed Decarboxylative Arylation Reactions.
Carrer A, et al.
Advanced Synthesis & Catalysis, 355(10), 2044-2054 (2013)
Nonpeptide Arginine Vasopressin Antagonists for Both V1A and V2 Receptors: Synthesis and Pharmacological Properties of 2-Phenyl-4'-((2, 3, 4, 5-tetrahydro-1H-1-benzazepin-1-yl) carbonyl) benzanilide Derivatives.
Matsuhisa A, et al.
Chemical & Pharmaceutical Bulletin, 45(11), 1870-1874 (1997)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico