472654
N-Boc-2-aminoacetaldehyde
95%
Sinónimos:
tert-Butyl N-(2-oxoethyl)carbamate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
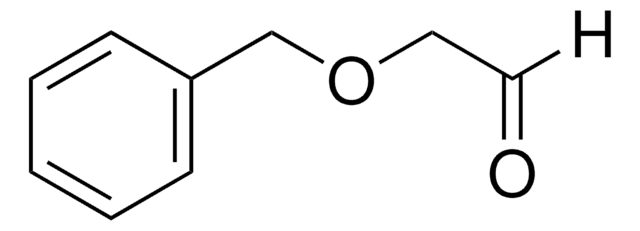
Fórmula lineal:
HCOCH2NHCO2C(CH3)3
Número de CAS:
Peso molecular:
159.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
95%
refractive index
n20/D 1.455 (lit.)
functional group
aldehyde
amine
storage temp.
−20°C
SMILES string
CC(C)(C)OC(=O)NCC=O
InChI
1S/C7H13NO3/c1-7(2,3)11-6(10)8-4-5-9/h5H,4H2,1-3H3,(H,8,10)
InChI key
ACNRTYKOPZDRCO-UHFFFAOYSA-N
Gene Information
human ... CTSK(1513)
General description
N-Boc-2-aminoacetaldehyde is an organic building block. It reacts with Horner-Wadsworth-Emmons (HWE) reagent to afford γ-aminobutyric acid (GABA)-derived α-keto amide/ester units.
Application
α-Methylenation of this amino aldehyde proceeds in a quick and efficient manner using a recently reported protocol involving formaldehyde and catalysis by either pyrrolidine proprionic acid or the dipeptide L-Pro-β-Ala.
Also used in a three-component synthesis of pyrrolidines involving 1,3-dipolar cycloaddition.
Also used in a three-component synthesis of pyrrolidines involving 1,3-dipolar cycloaddition.
N-Boc-2-aminoacetaldehyde may be employed in the following:
- As a starting reagent in the total synthesis of (+)-negamycin.
- Synthesis of (E)-ethyl 4-((tert-butoxycarbonyl)amino)but-2-enoate.
- Synthesis of 2,2′-bipyridine.
A building block in the synthesis of a protected pyrroloproline.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Efficient total synthesis of (+)-negamycin, a potential chemotherapeutic agent for genetic diseases.
Yoshio Hayashi et al.
Chemical communications (Cambridge, England), (20)(20), 2379-2381 (2008-05-14)
Herein, we describe an efficient strategy for the total synthesis of (+)-negamycin using commercially available achiral N-Boc-2-aminoacetaldehyde as starting material with 42% overall yield for a limited number of steps.
Claudia Karnthaler-Benbakka et al.
Angewandte Chemie (International ed. in English), 53(47), 12930-12935 (2014-08-01)
The development of receptor tyrosine-kinase inhibitors (TKIs) was a major step forward in cancer treatment. However, the therapy with TKIs is limited by strong side effects and drug resistance. The aim of this study was the design of novel epidermal
Anna Turetsky et al.
Scientific reports, 4, 4782-4782 (2014-04-25)
A number of Bruton's tyrosine kinase (BTK) inhibitors are currently in development, yet it has been difficult to visualize BTK expression and pharmacological inhibition in vivo in real time. We synthesized a fluorescent, irreversible BTK binder based on the drug
Diethyl [3-Cyano-2-Oxo-3-(Triphenylphosphoranylidene) propyl] phosphonate: A Useful Horner-Wadsworth-Emmons Reagent for alpha-Keto (Cyanomethylene)-triphenylphosphoranes from Carbonyl Compounds.
Lee K.
Bull. Korean Chem. Soc., 28(10), 1641-1641 (2007)
Anniina Erkkilä et al.
The Journal of organic chemistry, 71(6), 2538-2541 (2006-03-11)
A rapid and extremely convenient method for alpha-methylenation of aldehydes with aqueous formaldehyde is described. Two optimal catalytic systems are presented that allow short reaction times and afford the functionalized products in good to excellent yields (up to 99%) and
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![3-[(Benzyloxycarbonyl)amino]propionaldehyde 95%](/deepweb/assets/sigmaaldrich/product/structures/408/203/100fb0f0-7072-41be-b6e0-2857cdc324ee/640/100fb0f0-7072-41be-b6e0-2857cdc324ee.png)