389439
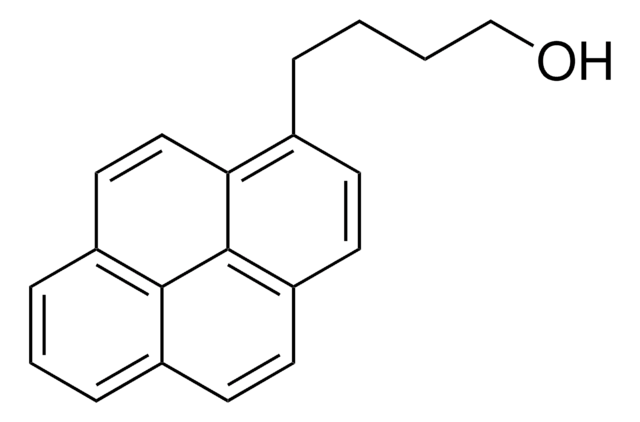
1-Pyrenemethanol
98%
Sinónimos:
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C17H12O
Número de CAS:
Peso molecular:
232.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
solid
mp
123-126 °C (lit.)
functional group
hydroxyl
SMILES string
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
InChI key
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Application
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
C D Sherman et al.
Carcinogenesis, 16(10), 2499-2506 (1995-10-01)
The promotional effect of phenobarbital and 1-hydroxymethyl-pyren on enzyme altered lesions in the rat liver were quantified within the framework of two separate multipath/multistage models. The experiment analyzed followed an initiation-promotion protocol in which female Wistar rats were initiated with
H Glatt et al.
Mutation research, 324(3), 111-114 (1994-07-01)
Previous studies demonstrated that the ion composition of the exposure medium may strongly influence the mutagenicity of many compounds in the liquid preincubation modification of the reversion assay with his- Salmonella typhimurium strains. Similar influences were now observed in the
H Glatt et al.
Environmental health perspectives, 88, 43-48 (1990-08-01)
Methylated polycyclic aromatic hydrocarbons are common in the human environment. Many of them are stronger carcinogens than their purely aromatic congeners. They may be metabolized to benzylic alcohols. We report here on biochemical and toxicological characteristics of 1-hydroxymethylpyrene (HMP), a
Bernhard H Monien et al.
Toxicology, 262(1), 80-85 (2009-06-02)
1-Methylpyrene (1-MP), an abundant alkylated polycyclic aromatic hydrocarbon, is activated by side-chain hydroxylation to 1-hydroxymethylpyrene (1-HMP) and subsequent sulfo-conjugation to electrophilic 1-sulfooxymethylpyrene (1-SMP). In rats, this activation mainly occurs in liver. 1-SMP may react with hepatic DNA or be exported
Ronny Kollock et al.
Biochemical pharmacology, 75(2), 527-537 (2007-10-09)
Alkylated polycyclic aromatic hydrocarbons can be metabolically activated via benzylic hydroxylation and sulphation to electrophilically reactive esters. However, we previously found that the predominant biotransformation route for the hepatocarcinogen 1-hydroxymethylpyrene (1-HMP) in the rat in vivo is the oxidation of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico







![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)