293768
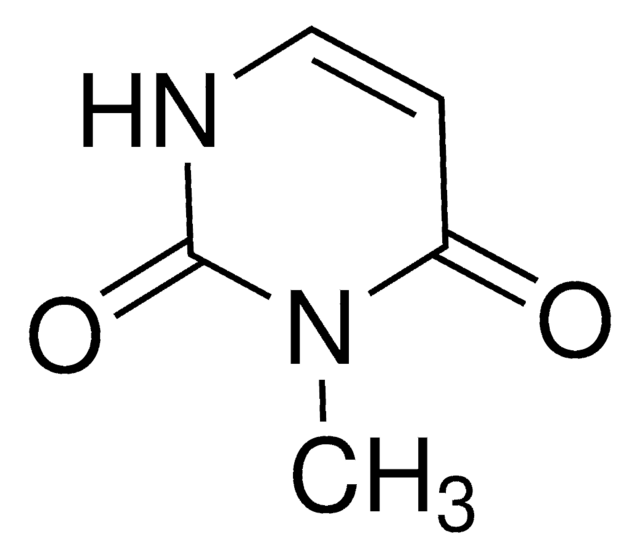
1-Methyluracil
99%
Sinónimos:
1-Methyl-2,4(1H,3H)-pyrimidinedione
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H6N2O2
Número de CAS:
Peso molecular:
126.11
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
99%
mp
236-238 °C (lit.)
solubilidad
1 M NaOH: soluble 50 mg/mL, clear, colorless
cadena SMILES
CN1C=CC(=O)NC1=O
InChI
1S/C5H6N2O2/c1-7-3-2-4(8)6-5(7)9/h2-3H,1H3,(H,6,8,9)
Clave InChI
XBCXJKGHPABGSD-UHFFFAOYSA-N
Descripción general
1-Methyluracil is of special importance in biochemistry, since uracil attaches ribose in ribonucleic acid (RNA) just precisely at the N1 atom. H-bond complex formation between 1-methyluracil and glycine has been investigated by theoretical calculations and FT-IR spectroscopy in Ar matrices. It forms 1:1 complexes with 9-ethyl-8-bromo-2,6-diaminopurine and the complex structure has been determined by three-dimensional X-ray diffraction methods.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Carc. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
R K McMullan et al.
Acta crystallographica. Section B, Structural science, 45 ( Pt 3), 270-276 (1989-06-01)
The crystal structure of 1-methylpyrimidine-2,4-dione (1-methyluracil, C5H6N2O2) has been determined at 15, 60 and 123 K from neutron diffraction data. Molecules lie in the eightfold special positions (symmetry m) of space group Ibam, with a = 13.213 (2), b =
V I Poltev et al.
Molekuliarnaia biologiia, 29(2), 365-375 (1995-03-01)
Monte Carlo simulation of hydration of keto and enol tautomers of 9-methylguanine (G) and 1-methyluracil (U) has been performed in relation to a possible role of tautomer transitions of DNA bases in mutagenesis. The comparison of the simulation results with
V I Poltev et al.
Journal of biomolecular structure & dynamics, 9(1), 101-111 (1991-08-01)
A number of nucleic acid base pairs and complexes between the bases and the amide group of acrylamide have been studied experimentally by using mass spectrometry and theoretically by the method of atom-atom potential function calculations. It has been found
E Sagstuen et al.
Radiation research, 149(2), 120-127 (1998-02-11)
Single crystals of the co-crystalline complex of 1-methyluracil and 9-ethyladenine were X-irradiated and studied using EPR, ENDOR and FSE spectroscopic techniques at 10 K. All together seven radicals were identified, and experimental evidence for at least one more species, as
Base-pairing configurations between purines and pyrimidines in the solid state. V. Crystal and molecular structure of two 1:1 hydrogen-bonded complexes, 1-methyluracil: 9-ethyl-8-bromo-2,6-diaminopurine and 1-ethylthymine: 9-ethyl-8-bromo-2,6--diaminopurine.
G Simundza et al.
Journal of molecular biology, 48(2), 263-278 (1970-03-14)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico





![6-Methylthieno[2,3-d]pyrimidine-2,4(1H,3H)-dione](/deepweb/assets/sigmaaldrich/product/structures/393/943/c932f315-dd4b-4939-aea6-646238005e48/640/c932f315-dd4b-4939-aea6-646238005e48.png)


